- EN - English
- CN - 中文
Micrografting in Arabidopsis Using a Silicone Chip
利用硅芯片进行拟南芥微嫁接
(*contributed equally to this work) 发布: 2021年06月20日第11卷第12期 DOI: 10.21769/BioProtoc.4053 浏览次数: 7949
评审: Masashi AsahinaDaisuke TodakaDaniel F. Caddell
Abstract
The micrografting technique in the model plant Arabidopsis has been widely used in the field of plant science. Grafting experiments have demonstrated that signal transductions are systematically regulated in many plant characteristics, including defense mechanisms and responses to surrounding environments such as soil and light conditions. Hypocotyl micrografting is a powerful tool for the analysis of signal transduction between shoots and roots; however, the requirement for a high level of skill for micrografting, during which small seedlings are microdissected and micromanipulated, has limited its use. Here, we developed a silicone-made microdevice, called a micrografting chip, to perform Arabidopsis micrografting easily and uniformly. The micrografting chip has tandemly arrayed units, each of which consists of a seed pocket for seed germination and a micro-path to hold hypocotyl. All micrografting procedures are performed on the chip. This method using a micrografting chip will avoid the need for training and promote studies of systemic signaling in plants.
Graphic abstract:
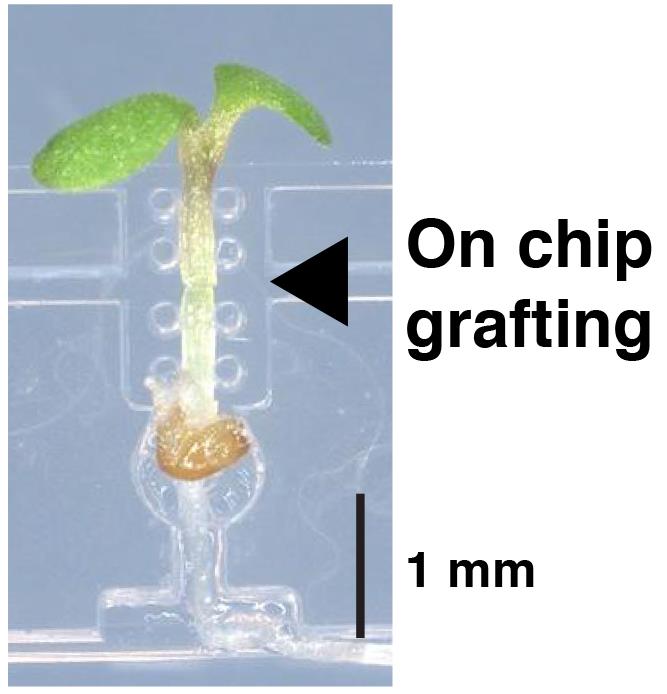
A silicone chip for easy grafting
Background
Grafting has served as a method to assemble two different types of plants, not only in agriculture but also in the field of plant science. Although plants do not have a nervous system, many studies using grafting experiments have demonstrated that plants properly integrate information from the entire body (Wang, 2011; Goldschmidt, 2014; Notaguchi and Okamoto, 2015; Thomas and Frank, 2019). In response to changes in their surrounding environments, plants generate information signals locally at the site of reception and transport signaling molecules systemically to transmit information. Inside plants, organ-to-organ communication is achieved via vascular tissues using a wide variety of molecules such as phytohormones, small RNAs, mRNAs, and proteins.
To study systemic signaling, especially between shoots and roots, micrografting, also known as seedling grafting, has been used. Micrografting is a method for assembling a scion and a rootstock that are cut out of seedlings from different plants. Since the first micrografting technique in the model plant Arabidopsis was demonstrated by Turnbull et al. (2002), micrografting has been widely used in a great number of studies and has contributed to the identification of molecules involved in systemic signaling such as flowering, defense against biotic infection, nutrient absorption and allocation, and drought stress response (Tsutsui and Notaguchi, 2017).
However, the micrografting technique requires dexterous operation and practice to achieve a high rate of success. Several papers have developed improved methods using modified procedures and equipment (Notaguchi et al., 2009), an agar-base support (Marsch-Martínez et al., 2013), and a fine pin to align the scion and the stock (Huang and Yu, 2015); nevertheless, micrografting remains challenging for beginners. To overcome the difficulties in conventional micrografting methods and make this technique accessible to those who study systemic signaling, we recently developed a microdevice made of silicone rubber, called a micrografting chip (Tsutsui et al., 2020). The device was fabricated by Micro-Electro Mechanical Systems using poly(dimethylsiloxane) (PDMS). The micrografting chip supports the manipulation of entire micrografting processes and facilitates the generation of uniformly grafted plants. This method has been applied in studies on plant biology using Arabidopsis mutants (Tsutsui et al., 2020; Shinozaki et al., 2020; Kurotani et al., 2020) and molecular mechanisms of grafting (Notaguchi et al., 2020). Here, we show the procedure for micrografting using a micrografting chip, which is slightly modified in its structure from Tsutsui et al. (2020) (Figure 1).
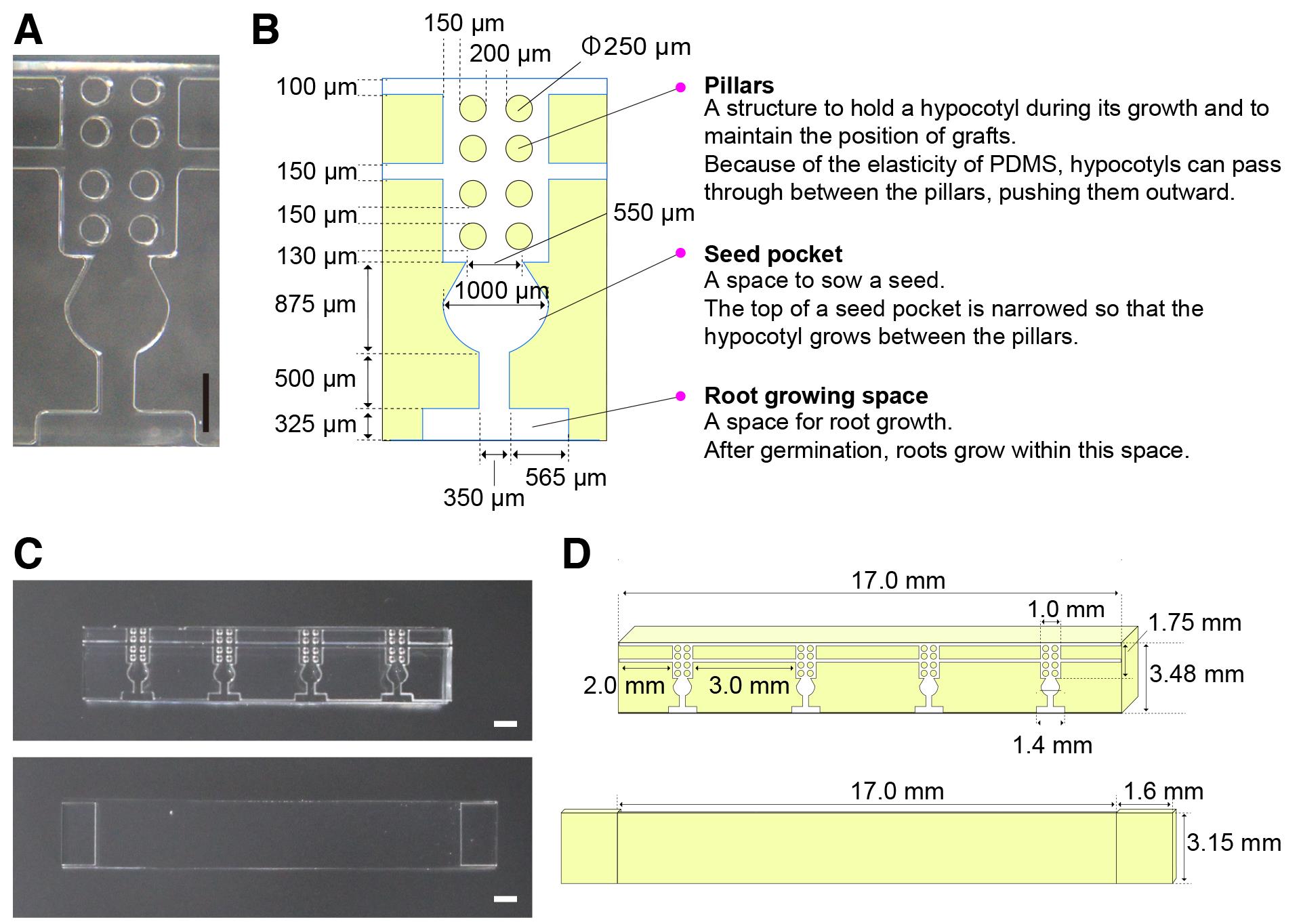
Figure 1. Structure of a micrografting chip. A. Image of one unit of the micrografting chip. B. Layout of the unit of the micrografting chip. C. Images of the micrografting chip (top) and its cover (bottom). D. Layouts of the micrografting chip (top) and its cover (bottom).
Materials and Reagents
Disposable gloves (Kimberly-Clark, catalog number: 52816)
Square Petri dishes with grid (Simport Scientific, catalog number: D210-16)
Petri dishes (ASNOL, catalog number: GD90-15)
1.5 ml tubes (Trefflab, catalog number: 96.07246.9.01)
Disposable surgical scalpel No.11 (KAI, catalog number: 511-A)
Disposable dropper 2 ml (Maruemu, catalog number: 0806-03)
Filter paper No.1, 70 mm (ADVANTEC, catalog number: 00011070)
Hybond-N+ membrane (GE Healthcare, catalog number: RPN119B)
Dissecting needle
Surgical tape (3M, catalog number: 1530-0)
Aluminum foil (UACJ)
KimWipes (CRECIA, catalog number: S-200)
Seeds of Arabidopsis thaliana
Note: Fresh seeds must be used. For successful micrografting, a scion and a stock at the same developmental stage are required. Old seeds show variation in the timing of seed germination and subsequent growth, hampering graft establishment. We used Arabidopsis thaliana ecotype Col-0 mainly, but other ecotypes can also be used.
Murashige-Skoog medium (Duchefa Biochemie, catalog number: M0221)
Sucrose (WAKO, catalog number: 190-00013)
Agar powder (Nacalai tesque, catalog number: 01028-85)
MES hydrate (Sigma-aldrich, catalog number: M2933)
76.9-81.4%(v/v) ethanol (KANEICHI, catalog number: 4987556210311)
Potassium hydroxide (WAKO, catalog number: 168-21815)
Bleach (KAO, catalog number: 017598)
1 N KOH (see Recipes)
1% agar plates (see Recipes)
2% agar plates (see Recipes)
Hybond-N+ membrane (GE Healthcare, catalog number: RPN119B)
0.4% agar for resuspension of sterilized seeds (see Recipes)
Filter paper (see Recipes)
Sterilized water (see Recipes)
Bleach solution (see Recipes)
Sterilized seeds (see Recipes)
Equipment
pH meter (HORIBA, catalog number: F-51)
Pipette P20 (INMEDIAM, catalog number: FA10003P)
Micrografting chip (Bio Medical Science Inc., catalog number: BGA-GRC020) (Figure 1)
Note: We used the micrografting chips as disposable. The purchased chips are sterilized and ready to use after opening the package.
Super clean zone creator (Koken, catalog number: KOACH T 500-F)
Dissecting microscope (OLYMPUS, catalog number: SZX10)
Precision tweezers (Inox-biology, catalog number: 11252-00)
Vannas spring scissors (Fine Science Tools, catalog number: 15019-10)
Plant growth chamber (Biotron, catalog number: LPH-411S)
Bio-clean bench (AIRTECH, BLB-1306)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Tsutsui, H., Kawakatsu, Y. and Notaguchi, M. (2021). Micrografting in Arabidopsis Using a Silicone Chip. Bio-protocol 11(12): e4053. DOI: 10.21769/BioProtoc.4053.
- Notaguchi, M., Kurotani, K. I., Sato, Y., Tabata, R., Kawakatsu, Y., Okayasu, K., Sawai, Y., Okada, R., Asahina, M., Ichihashi, Y., Shirasu, K., Suzuki, T., Niwa, M. and Higashiyama, T. (2020). Cell-cell adhesion in plant grafting is facilitated by β-1,4-glucanases. Science 369(6504): 698-702.
分类
植物科学 > 植物生理学 > 表型分析
植物科学 > 植物分子生物学 > RNA > RNA 检测
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link