- EN - English
- CN - 中文
Objective Quantitation of Focal Sweating Areas Using a Mouse Sweat-assay Model
小鼠汗液分析模型对局部出汗区域的客观定量
发布: 2021年06月05日第11卷第11期 DOI: 10.21769/BioProtoc.4047 浏览次数: 3746
评审: Karem A CourtArvind Kumar Singh ChandelKuo-Ching "KC" Mei
Abstract
In vivo sweat quantitation assays are required for the development of drugs for the management of focal hyperhidrosis before clinical trials; however, in vivo assays, particularly mouse models, are rare. Even in sweat assays using mice, sweating is quantitated by manually counting the number of sweating spots, which can contribute to various errors owing to arbitrary judgment. In this study, we developed a mouse sweat-assay model and a method for quantitating the amount of sweating to remove possible errors. The use of the iodine–starch test in the castor oil-covered hind footpad skin of anesthetized mice resulted in the sweating area being stained blue-black. After the anesthesia and treatment with drugs (pilocarpine, glycopyrrolate, botulinum neurotoxin, myricetin, and myricetin-loaded lipid nanoparticles), the remaining area of the footpad skin was eliminated from the acquired footpad images using ImageJ. Blue pixels extracted from the footpad image are automatically adjusted using the Phansalkar method, where the percentage of the blue area was determined based on the whole hind footpad skin area, finally indicating the percentage of the sweating area. Using this mouse model and analysis for sweat assays, a clear difference between the control group and antiperspirant-administered group was observed with respect to the sweating area % with no error. In conclusion, this assay can be used as a preclinical tool to screen potential antiperspirant drugs.
Graphic abstract:
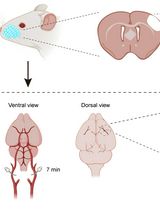
Overview of the mouse-model sweat assay and objective quantitation of the focal sweating area
Background
Numerous populations – nearly 3% of people aged 25-64 years – experience focal hyperhidrosis, an idiopathic abnormality characterized by hyper-sweating concentrated in certain regions of the body, e.g., palms, soles, face, and armpits (Haider et al., 2005). It interferes with daily activities; therefore, this condition poses substantial emotional, psychological, social, and professional burden (Strutton et al., 2004) and even leads to social anxiety disorders (Solish et al., 2007). During the development of drugs or medications for the treatment of focal hyperhidrosis, rational assays for evaluating sweating are necessary to evaluate excessive perspiration symptoms. Several tests used in clinical practice and research studies are not sensitive enough to detect subtle perspiration changes resulting from medications (Provitera et al., 2010). The thermoregulatory sweat test stimulates entire body sweating, including central and peripheral components, but does not localize the sweating lesion site (Fealey et al., 1989). Simple colorimetric procedures, such as the Neuropad test, evaluate sweating abnormalities but do not provide detailed analysis (Quattrini et al., 2008). In addition, abundant in vivo preclinical data are necessary for the verification of anti-perspiration efficacy before clinical trials; however, studies, particularly using mouse models, are rare.
The iodine-starch test is a well-known chemical reaction between starch and iodine, which instantly produces an intense blue-black color. The test was first described in 1814 (Colin et al., 1814) and first applied in 1928 as a medical test for visualizing sudomotor function (perspiration or sweating) (Minor, 1928). Polyiodides complexed with the amylose helix in starch form infinite polyiodide homopolymers and produce the blue-black color, whereas non-ionized iodine dissolved in nonpolar solvents and individual iodide (I−) do not react with starch (Madhu et al., 2016). The triiodide (I3−) ion is the simplest polyiodide, which is yellow-to-brown in aqueous conditions with no starch. In addition, the blue-black color of the triiodide-starch complex is so deep, which can also be detected visually when the concentration of iodine is as low as 2 × 10−5 M at 20°C. Consequently, the iodine-starch test has been used as a well-established diagnostic tool to evaluate underactive (hypohidrosis) and overactive (hyperhidrosis) sweating (Chia et al., 2013).
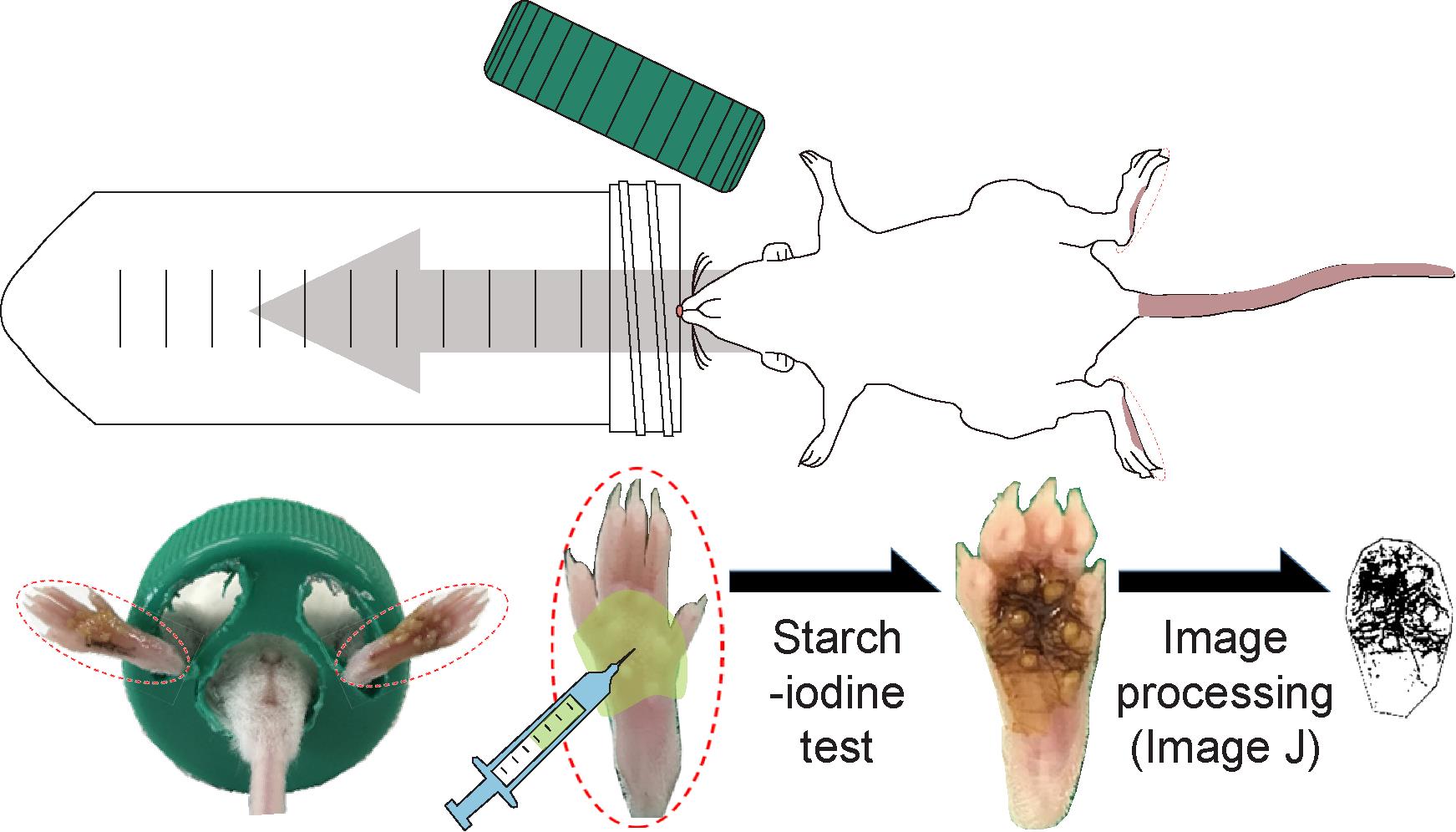
Even in the mouse sweat assays, various errors may occur owing to arbitrary judgment because the number of sweating spots, attributed to the iodine-starch reaction, is manually counted to quantitate the sweating score (Nejsum et al., 2002; Liu et al., 2017). For the data analysis, image-processing using ImageJ, a free software provided by the National Institutes of Health (NIH), can be utilized to eliminate possible arbitrary judgment and errors. In this protocol, we generated a mouse sweat-assay model and used ImageJ for image analysis (Ban et al., 2020). The sweating area on the skin of hind footpads of anesthetized mice was stained blue-black using an iodine-starch test. The blue-black pixels were extracted from the footpad images obtained, adjusted using the Auto Local Threshold function in ImageJ, and measured to the area fraction (sweating area %). Using the present protocol, a clear difference between the control and the antiperspirant-administered mice was observed with respect to the sweating area % with no error. Moreover, whether expected antiperspirants exploit either circulatory-systematic or focal anti-perspiration efficacy can also be assessed using this protocol.
Materials and Reagents
Pipet tips, 20 µl, 200 µl, and 1,000 µl (Gilson, catalog numbers: F161671, F1739311, and F161451, respectively)
0.2 μm syringe filter
Conical tube with lid, 50 ml (SPL Life Sciences, catalog number: 50050)
Microtubes, 200 µl and microtube 1.5 ml (Sigma-Aldrich, catalog number: Z374873; Eppendorf, catalog number: EP0030125150)
Sterilized needles for syringe, 17 gauge (Korea vaccine)
Sterilized syringe filter (Sartorius, catalog number: 17764-ACK)
Sterilized syringes for general use, 1 and 50 ml (Korea vaccine)
Mice (6-week-old Institute of Cancer Research, weighing 25-35 g; Koatec Co., Pyeongtaek, Korea)
Myricetin (Sigma-Aldrich, catalog number: M6760)
Glyceryl tristearate (Sigma-Aldrich, catalog number: T5016)
Glyceryl trioctanaote (Sigma-Aldrich, catalog number: T9126)
Brij S100 (Sigma-Aldrich, catalog number: 466387)
Pluronic P-123 (Sigma-Aldrich, catalog number: 435465)
Pilocarpine hydrochloride (pilocarpine) (Tokyo Chemical Industry, catalog number: P0434)
Glycopyrrolate bromide (glycopyrrolate) (Tokyo Chemical Industry, catalog number: G0392)
Neuronox 100U (botulinum neurotoxin type A, BoNT/A) (Medytox)
Alfaxalone solution, 10 mg/ml (Alfaxan) (Alfaxan Multidose, Jurox Animal Health)
Xylazine hydrochloride solution, 23.32 mg/ml (Rumpun, Bayer)
Starch (Millipore, catalog number: 101252)
Castor oil (Sigma-Aldrich, catalog number: 259853)
Iodine (Sigma-Aldrich, catalog number: 207772)
Ethanol (Sigma-Aldrich, catalog number: 02891)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: 276855)
HyClone phosphate-buffered saline, pH 7.4 (PBS) (GE Healthcare, catalog number: SH30256.01)
Rumpun solution (see Recipes)
Anesthesia solution (see Recipes)
Pilocarpine solution (see Recipes)
Glycopyrrolate solution (see Recipes)
Myricetin solution (see Recipes)
Myricetin-loaded lipid nanoparticles (M-LNPs) (see Recipes)
Starch solution (see Recipes)
Iodine solution (see Recipes)
Equipment
Pipets (20, 200, and 1,000 µl) (Gilson, catalog numbers: FA10003M, FA10005M, and FA10006M, respectively)
Electric pharynx
Pioneer analytical balance, capacity 320 g (Ohaus, model: PX323E)
Cages for stabilizing between the time of anesthetic injection and complete anesthesia
Paintbrushes to apply iodine and starch solutions on the hind footpads of mice
Digital camera (Canon, model: EOS 800D)
Mouse restrainers were prepared by making holes in the plastic wall of conical tubes and the lid using an electric pharynx (Figure 1)
Software
ImageJ (NIH, http://rsb.info.nih.gov/ij/) for image-processing and determination of the sweating area %
SigmaPlot 10.0 Windows version (IBM Co.) for drawing graphs
SPSS Statistics version 23.0 software (IBM Co.) for the statistical analyzes
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Ban, C. and Kweon, D. (2021). Objective Quantitation of Focal Sweating Areas Using a Mouse Sweat-assay Model. Bio-protocol 11(11): e4047. DOI: 10.21769/BioProtoc.4047.
分类
神经科学 > 感觉和运动系统 > 动物模型
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link