- EN - English
- CN - 中文
Single-Molecule Studies of Membrane Receptors from Brain Region Specific Nanovesicles
脑区特异性纳米泡膜受体的单分子研究
发布: 2021年05月20日第11卷第10期 DOI: 10.21769/BioProtoc.4018 浏览次数: 5964
评审: Shalini Low-NamAnca Flavia SavulescuAnonymous reviewer(s)
Abstract
Single molecule imaging and spectroscopy are powerful techniques for the study of a wide range of biological processes including protein assembly and trafficking. However, in vivo single molecule imaging of biomolecules has been challenging because of difficulties associated with sample preparation and technical challenges associated with isolating single proteins within a biological system. Here we provide a detailed protocol to conduct ex vivo single molecule imaging where single transmembrane proteins are isolated by rapidly extracting nanovesicles containing receptors of interest from different regions of the brain and subjecting them to single molecule study by using total internal reflection fluorescence (TIRF) microscopy. This protocol discusses the isolation and separation of brain region specific nanovesicles as well as a detailed method to perform TIRF microscopy with those nanovesicles at the single molecule level. This technique can be applied to study trafficking and stoichiometry of various transmembrane proteins from the central nervous system. This approach can be applied to a wide range of animals that are genetically modified to express a membrane protein-fluorescent protein fusion with a wide range of potential applications in many aspects of neurobiology.
Graphic abstract:
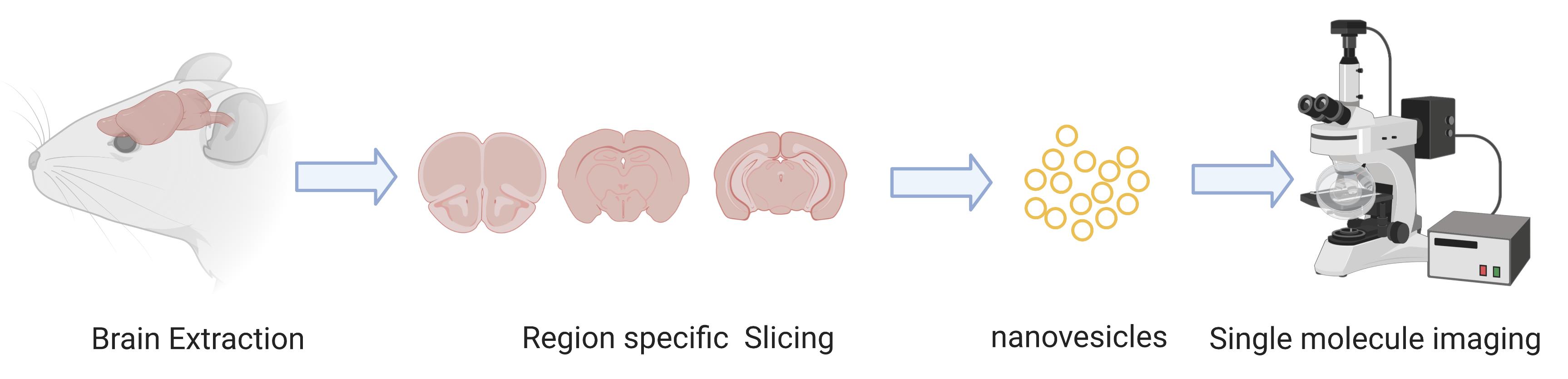
EX vivo single molecue imaging of membrane receptors
Background
Single molecule imaging techniques have emerged as powerful methods to study the structure, function, and interactions of biomolecules (Joo et al., 2008; Choi et al., 2019). While ensemble measurements provide important information about the average population, single molecule methods can be used to evaluate the heterogeneity within this population revealing differences in structural and functional states (Lee et al., 2020). Single molecule methods have already been used in a diverse range of applications including measurements of protein and ligand interaction, conformational changes in proteins, and ion channel function (Xia et al., 2013; Martinac, 2017; Fu et al., 2019). These methods have provided powerful ways to characterize individual cellular constituents and single molecules in vitro.
Despite their successful implementations in vitro, single molecule methods for in vivo studies have been particularly challenging. Major challenges for in vivo studies include the heterogenous nature of cellular components, the difficulties associated with light penetration in thick tissues, and the need to isolate a single species from the complex environment within a living system (Fu et al., 2019). In addition, the concentration of protein of interest in some cases is much higher in the native environment than the optimum concentration required for single molecule imaging (Richards et al., 2012). Additionally, membrane proteins are not spatially isolated in a single location within the cellular environment and instead diffuse across a wide region, complicating single molecule studies. This makes isolation and separation of the molecule of interest a key aspect of any ex vivo single molecule imaging protocol. This protocol details an approach to address these challenges, by utilizing nanoscale vesicles generated from primary tissue as a way of isolating and immobilizing membrane proteins. We demonstrate this ex vivo single molecule imaging approach using knock-in mice containing a fluorescent protein labeled nicotinic acetylcholine receptor (nAChRs). Nicotinic acetylcholine receptors (nAChRs) are transmembrane proteins which respond to and can be upregulated during nicotine exposure (Cano et al., 2020). Their trafficking and stoichiometry have also shown to be altered by nicotine (Srinivasan et al., 2011). In this protocol, we used a mouse model where the nAChR α4 subunit is labelled with green fluorescence protein (GFP).
We believe this protocol can be widely applied to virtually any mouse model that focuses on a transmembrane protein that is genetically labeled with a fluorescent protein. This protocol will enable researchers to extend single molecule studies beyond common in vitro approaches by performing ex vivo characterization of physiological changes occurring in live animals at the individual protein level. Specifically, this protocol describes a detailed method to isolate nanovesicles from specific regions of the mouse brain in order to perform single molecule imaging to characterize membrane receptors. This technique can be used to understand brain region-specific structural and functional changes in various animal models. An advantage of this approach is that during the isolation of the vesicles, transmembrane receptors maintain their structural integrity because they remain in their endogenous membrane in the nanovesicles. This allows one to characterize their structural assembly as well as their functional properties as in their native physiological environment (Fu et al., 2019). This single molecule protocol which involves sample preparation and labelling along with Total Internal Reflection Fluorescence (TIRF) Microscopy should be immediately applicable to a wide range of existing mouse models already in use for other neuroscience applications.
Materials and Reagents
Tygon tubing (Garinger, Item number: 9MH86)
1 ml plastic syringe (Grainger, Item number: 19G334, Thermo Fisher Scientific, catalog number: NC0786233)
Syringe needles (Air-Tite, catalog number: 161028)
50 ml centrifuge tubes (VWR, catalog number: 89039-658)
5 ml Serological pipettes (VWR, catalog number: 89130-896)
15 ml centrifuge tubes (VWR, catalog number: 89039-666)
1.5 ml Flex tubes Eppendorf (VWR, catalog number: 20901-421)
Ultra-Clear ultracentrifuge tubes (Beckman Coulter, catalog number: 344061)
Dounce Tissue Grinder 7ml (Omni International, catalog number: 07-357542)
Glass bottom dish (MatTek, catalog number: P35G-1.5-14-C)
Fatal Plus Solution (Drugs.com, product number: V.P.L. 9373, NDC number: 0298-9373-68)
Protease inhibitor mini tablet (Thermo Fisher Scientific, catalog number: A32955)
Sucrose (VWR, catalog number: 97063-788)
HEPES (Fisher Scientific, catalog number: BP310-500)
Ice
Milli-Q Water (Fisher Scientific, catalog number: QGARD00D2)
1× PBS buffer (VWR, catalog number: 97062-948)
HCl (VWR, catalog number: 470301-260)
NaOH ((VWR, catalog number: BDH7225-4)
Biotin-PEG-Silane (Laysan Bio, Item number: Biotin-PEG-SIL-3400-500mg)
Neutravidin protein (Thermo Fisher Scientific, catalog number: 31000)
Biotinylated anti-GFP antibody (Rockland antibodies and assays, item number: 600-406-215)
Sucrose buffer/Homogenization buffer, pH 7.4 (see Recipes)
Fatal Plus Working Solution (see Recipes)
Equipment
Smooth fine forceps (Electron Microscopy Sciences, 1209K43, catalog number: 72911-6)
Surgical scissors (Medline, catalog number: MDS0838410)
Operating scissors (Stoelting, catalog number: 52138-51)
Flat tip forceps (Fisher Scientific, catalog number: 12-000-123)
Ice bucket (VWR, catalog number: 10146-202)
Adult Mouse Brain Slicer Matrix (Zivic Instruments, catalog number: BSMAS002-1)
1 ml pipettor (Gilson, catalog number: F123602)
Pipet controller (VWR, catalog number: 613-4442)
Autoclave (Steris Amsco Eagle, serial no. 096899 SAW)
Perfusion pump (Grainger, catalog number: 2688)
Centrifuge (Beckman Coulter, model: Allergra® X-22R; Rotor: Beckman Coulter, model: SX4250)
Ultracentrifuge (Beckman Coulter, model: L-60)
Swing bucket rotor (Beckman Coulter, model: SW 28)
Fixed angle rotor (Beckman Coulter, model: 70 Ti)
Belly dancer or orbital shaker (Fisher Scientific, catalog number: 15-453-211)
Sonicator (Amsco Reliance Sonic 550)
Fume Hood (Supreme Air LV, Kewaunee Scientific, VWR, catalog number: 97006-002) or an air compression system
Oxygen plasma cleaner (Harrick Plasma, catalog number: PDC-32G)
Vortex (VWR, catalog number: 10153-836)
4 °C fridge
-20 °C freezer
A TIRF-capable microscope (Olympus, model: IX2-ZDC2, serial number: 0F83094)
Software
Metamorph (Available on Olympus Microscope)
ImageJ/FIJI (Freely available from NIH)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Aryal, S. P., Fu, X., Masud, A. A., Neupane, K. R. and Richards, C. I. (2021). Single-Molecule Studies of Membrane Receptors from Brain Region Specific Nanovesicles. Bio-protocol 11(10): e4018. DOI: 10.21769/BioProtoc.4018.
分类
生物物理学 >
神经科学 > 基础技术 > 受体-受体互作
生物化学 > 蛋白质 > 单分子活性 > 成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












