- EN - English
- CN - 中文
A Potent Vaccine Delivery System
一个有效的疫苗输送系统
发布: 2021年04月05日第11卷第7期 DOI: 10.21769/BioProtoc.3973 浏览次数: 6444
评审: Pawel Paweł KafarskiShyam SrivatsAnonymous reviewer(s)
Abstract
Most vaccines require co-delivery of an adjuvant in order to generate the desired immune responses. However, many currently available adjuvants are non-biodegradable, have limited efficacy, and/or poor safety profile. Thus, new adjuvants, or self-adjuvanting vaccine delivery systems, are required. Here, we proposed a self-adjuvanting delivery system that is fully defined, biodegradable, and non-toxic. The system is produced by conjugation of polyleucine to peptide antigen, followed by self-assembly of the conjugate into nanoparticles. The protocol includes solid-phase peptide synthesis of the vaccine conjugate, purification, self-assembly and physicochemical characterization of the product. Overall, this protocol describes, in detail, the production of a well-defined and effective self-adjuvanting delivery system for peptide antigens, along with tips for troubleshooting.
Keywords: Poly (hydrophobic amino acid) (聚(疏水氨基酸))Background
Peptide subunit vaccines, which use the small antigen fragments (epitopes) to trigger protective immune responses against infectious diseases, are one of the most promising vaccine technologies to have emerged in recent decades (Skwarczynski and Toth, 2016; Malonis et al., 2020). However, as peptides, themselves, are always poorly immunogenic, they need to be co-administered with an adjuvant (immune stimulator) and/or delivery system (Azmi et al., 2014; Nevagi et al., 2018). Currently, only a few options exist when it comes to adjuvants that are safe enough to be administered to humans. While more numerous in options, experimental adjuvants are often poorly defined, toxic, or have limited efficacy (Shi et al., 2019). One of the most recent strategies developed to deliver vaccines utilizes nanostructures with self-adjuvanting properties (Skwarczynski and Toth, 2014). Self-assembling polymers, in particular, have been widely investigated (Zhao et al., 2017; Nevagi et al., 2019). However, the structures of these polymers are not fully defined (number of units, stereochemistry) and, therefore, batch variability may affect vaccine activity and safety profile.
We have conceptualized, designed, and developed a new vaccine adjuvant/delivery system to overcome the disadvantages outlined above. This system is based on fully-defined and biodegradable polymers built from our own natural hydrophobic amino acids. The lead vaccine candidate produced based on this system was able to stimulate the production of highly opsonic antibodies against six clinical isolate strains of group A streptococcus in mice (Skwarczynski et al., 2020). The compound was more efficient than the powerful, but toxic, “gold standard” Complete Freund’s Adjuvant and did not induce undesired inflammatory responses. The strategy to deliver antigenic epitopes attached to self-adjuvanting amino acid-based polymer described here offers an attractive, safe alternative to conventional vaccine adjuvants. Importantly, this approach can be fully customized to match the properties of the antigen of choice. The procedure on how to produce this vaccine candidate (Figure 1) is presented here, with reference to the published vaccine study (Skwarczynski et al., 2020). Notes provide additional helpful information.
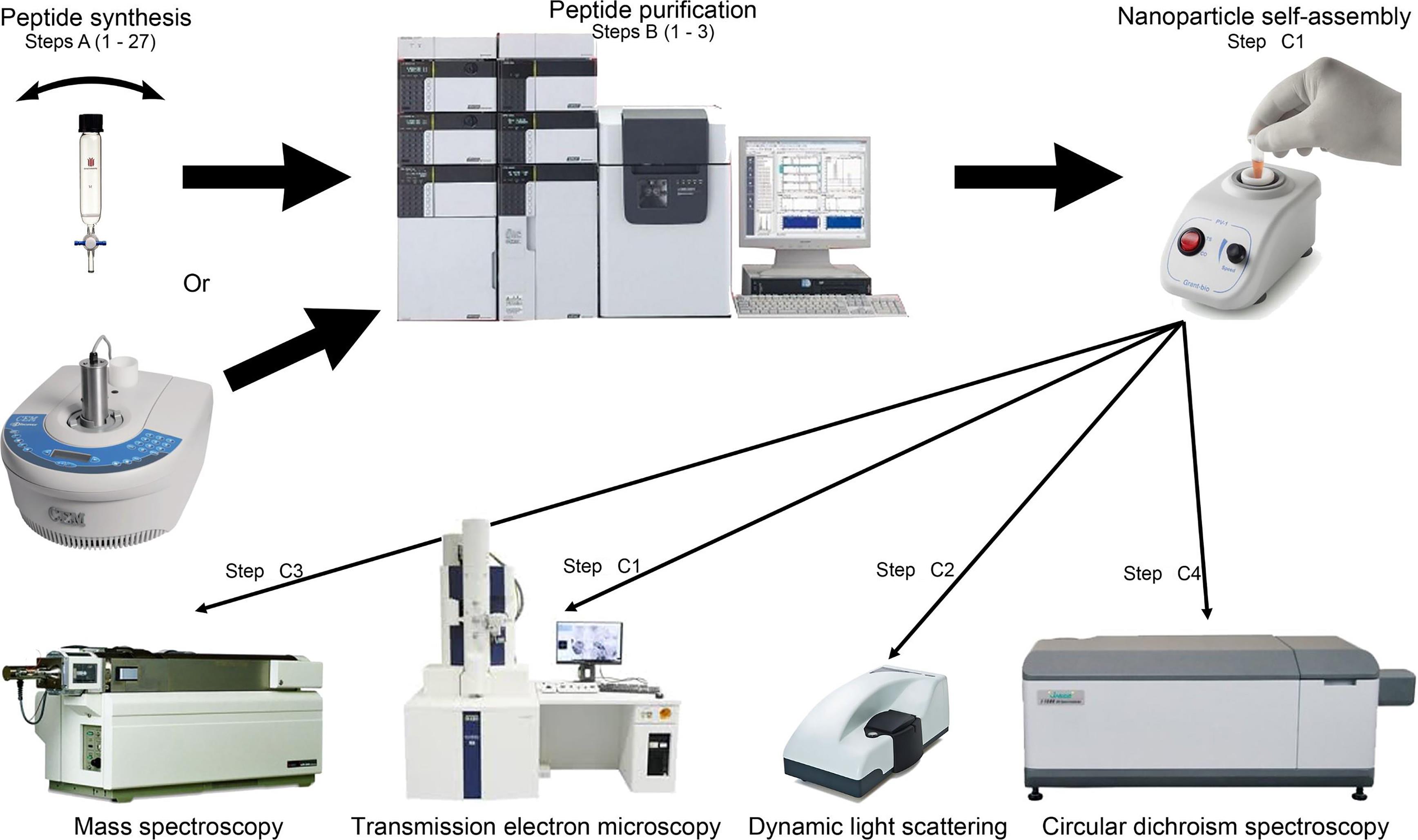
Figure 1. Flowchart of vaccine candidate synthesis, purification and characterization steps
Materials and Reagents
Note: All chemicals should be analytical grade, unless stated otherwise.
Vaccine candidate synthesis
Chemical resistance gloves (Ansell, catalog number: 02-100 )
Rink amide p-methyl-benzhydrylamine hydrochloride (pMBHA·HCl) resin (substitution: 0.59 mmol/g; 100-200 mesh; Peptides International, catalog number: RMB-1045-PI )
N,N-dimethylformamide (DMF; Merck, catalog number: 227056 ) (see Note 1)
N,N-diisopropylethylamine (DIPEA; 6.2 equivalent; Merck, catalog number: 387649 )
Trifluoroacetic acid (TFA; Merck, catalog number: 302031 )
Butyloxycarbonyl (Boc)–protected l-amino acids (0.84 mmol/g; 4.2 equivalent; Novabiochem Merck Chemicals and Mimotopes)
2-(7-Aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HATU; 0.5 M; 4 equivalent; Mimotopes, catalog number: 148893-10-1 ) solution: 9.5 g HATU in 50 ml DMF (store solution at 0 °C for no longer than 1 week after preparation) (see Note 2)
Capping solution: 5% acetic anhydride (Sigma-Aldrich, catalog number: 320102 ), 5% DIPEA, and 90% DMF (v/v/v)
Dichloromethane (DCM; Merck, catalog number: 270997 )
Piperidine deprotection solution: 20% piperidine (Sigma-Aldrich, catalog number: 8.22299 ) and 80% DMF (v/v)
Methanol (Merck, catalog number: 34860 )
p-Cresol (Sigma-Aldrich, catalog number: C85751 )
Hydrofluoric acid (HF; Ghc Gerling, Holz & Co. Handels gmbh, catalog number: 3100 , Hydrogen Fluoride [99.95%])
Diethyl ether (Sigma-Aldrich, catalog number: 91238 )
n-hexane (Merck, catalog number: 1.04367 )
Acetonitrile (Merck, catalog number: 271004 )
Endotoxin-free Milli-Q water (sensitivity of 18.2 MΩ.cm at 25 °C and total organic content below 5 parts per billion)
Solvent A: 100% Milli-Q water and 0.1% TFA (v/v; solution can be stored at room temperature for up to 3 months)
Solvent B: 90% acetonitrile, 10% Milli-Q water, and 0.1% TFA (v/v/v; solution can be stored at room temperature for up to 3 months)
Vaccine candidate purification
Phenex syringe filter (0.45 µm; Phenomenex, catalog number: AF3-3107-52 )
Reagents listed previously (Solvent A and B)
Vaccine candidate characterization
Disposable capillary cuvettes (Malvern Analytical, model: DTS1070 )
Whatman filter paper (Merck, catalog number: WHA1005090 )
Phosphate-buffered saline (PBS; ThermoFisher Scientific, catalog number: 10010031 )
Phosphotungstic acid stain (2%): 2 mg phosphotungstic acid hydrate (Sigma-Aldrich, catalog number: P4006-25G ) in 100 ml Milli-Q water (stir for 1 h, then filter; solution can be stored at 2-8 °C for up to 3 months)
Equipment
Vaccine candidate synthesis
Laboratory glassware
CEM Discover Solid Phase Synthesis (SPS) reactor (CEM Corporation, model: Discover SPS ; see Software 1) (see Note 3)
Peptide synthesis vessel (CEM Corporation, catalog number: 170897 ) (see Note 4)
Glass peptide synthesis vessel (Sigma-Aldrich, catalog number: Z41,850-1 )
CEM Discovery SPS vacuum manifold filtration apparatus (CME Corporation, catalog number: 167993 ) (see Note 4)
Scintillation vials (Merck, catalog number: DWK986568 )
Vortex mixer (Phoenix Instruments, model: RS-VA 1) or sonicator (Baranson Ultrasonicator Corporation, catalog number: 2510E-MTH )
Rotary mixer (Ratek Instruments, catalog number: RSM7DC )
Desiccator
Hydrofluoric acid (HF)-reaction apparatus (including HF reaction vessel) for peptide cleavage from the resin (refer to Jadhav et al., 2020)
Alpha 2-4 LD freeze dryer (John Morris Scientific, catalog number: 101521 )
Vaccine candidate purification
Shimadzu preparative reverse-phase HPLC (RP-HPLC) instrument (Shimadzu, models: LC-20AP × 2 , CBM-20A , SPD-20A , FRC-10A ) with a 20.0 ml/min flow rate (see Software 2)
Vydac C4 (Hichrom, catalog number: 214TP54 , 5 µm, 4.6 × 250 mm; and 214TP1022 , 10 µm, 22 × 250 mm) or C8 columns (Hichrom, catalog number: 208TP54 ; 5 μm, 4.6 × 250 mm)
Perkin-Elmer- Sciex API3000 electrospray ionization mass spectrometry (ESI-MS) instrument (Applied Biosystems/MDS Sciex, model: Sciex API3000 ; see Software 3)
Shimadzu analytical RP-HPLC instrument (Shimadzu, models: DGU-20A5 , LC-20AB , SIL-20ACHT , SPD-M10AVP ) with a 1.0 ml/min flow rate (see Software 2)
Vaccine candidate characterization
Malvern Zetasizer dynamic light scattering (DLS; Malvern Instruments, model: Nano ZS ; see Software 4)
JEM-1010 transmission electron microscope (TEM; JEOL, see Software 5)
Carbon-coated copper grids (Pure Carbon Film 200 mesh, Ted Pella, catalog number: 01840-F )
Jasco J710 circular dichroism (CD) spectrometer (JASCO Corporation, model: J710 ; see Software 6)
CD 1.0 mm cell (Starna, catalog number: 21/Q/1/CD )
Software
SynergyTM (CME Corporation, North Carolina, USA, www.cemsynthesis.com)
LabSolutions (Shimadzu, Kyoto, Japan, www.shimadzu.com)
Analyst® 1.6 (Applied Biosystems/MDS Sciex, Toronto, Canada, www.sciex.com)
Malvern Zetasizer Analyzer 6.2 (Malvern Instruments, Worcestershire, UK, www.malvernpanalytical.com)
Olympus Soft Imaging Solutions (Olympus Corporation, Tokyo, Japan, www.olympus-global.com)
Spectra ManagerTM (JASCO Corporation, Tokyo, Japan, www.jascoinc.com)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Zhao, G., Azuar, A., Toth, I. and Skwarczynski, M. (2021). A Potent Vaccine Delivery System. Bio-protocol 11(7): e3973. DOI: 10.21769/BioProtoc.3973.
- Skwarczynski, M., Zhao, G., Boer, J., Ozberk, V., Azuar, A., Cruz, J. G., Giddam, A. K., Khalil, Z., Pandey, M., Shibu, M., et al. (2020). Poly(amino acids) as a potent self-adjuvanting delivery system for peptide-based nanovaccines. Sci Adv 6: eaax2285.
分类
免疫学 > 粘膜免疫学 > 疫苗佐剂
微生物学 > 体内实验模型 > 细菌
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link