- EN - English
- CN - 中文
Cranioplastic Surgery and Acclimation Training for Awake Mouse fMRI
清醒小鼠fMRI的颅成形手术及适应训练
发布: 2021年04月05日第11卷第7期 DOI: 10.21769/BioProtoc.3972 浏览次数: 3665
评审: Miao HeRuiqi WuAnonymous reviewer(s)
Abstract
MRI is a promising tool for translational research to link brain function and structure in animal models of disease to patients with neuropsychiatric disorders. However, given that mouse functional MRI (fMRI) typically relies on anesthetics to suppress head motion and physiological noise, it has been difficult to directly compare brain fMRI in anesthetized mice with that in conscious patients. Here, we developed a new system to acquire fMRI in awake mice, which includes a head positioner and dedicated radio frequency coil. The system was used to investigate functional brain networks in conscious mice, with the goal of enabling future studies to bridge fMRI of disease model animals with human fMRI. Cranioplastic surgery was performed to affix the head mount and the cupped-hand handling method was performed to minimize stress during MRI scanning. Here we describe the new mouse fMRI system, cranioplastic surgery and acclimation protocol.
Graphic abstract:
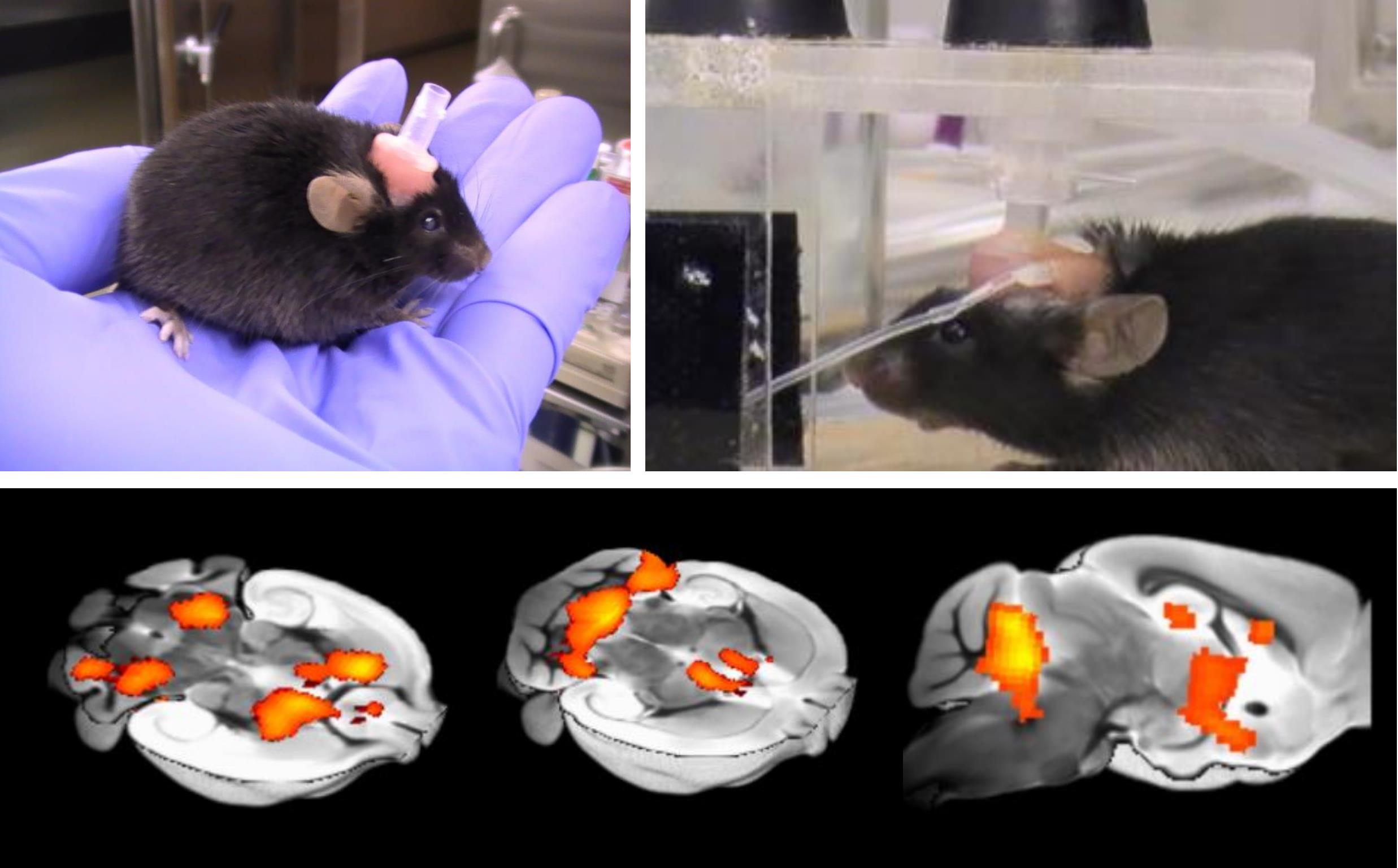
Awake fMRI system to investigate the neuronal activity in awaked mice.
Background
High-field mouse fMRI is an important translational tool to bridge the gap between invasive research in mouse models of neuropsychiatric diseases and clinical research in patients. However, compared to human fMRI, mouse fMRI studies are typically limited due to anesthesia, which is necessary to suppress body motion and stress during fMRI acquisition. Performing mouse fMRI studies under light anesthesia can ameliorate body motion, but the extent to which functional brain networks and connectivity are influenced by light anesthesia remains unclear. In general, small doses of dexmedetomidine, medetomidine, isoflurane or a mixture of these anesthetics are commonly used to achieve light anesthesia to investigate brain function in rodents (Grandjean et al., 2014; Bukhari et al., 2017; Tsurugizawa et al., 2019 and 2020a). However, these anesthetics induce not only suppression of consciousness (Munglani et al., 1993) but also vasoactive modulation of neurovascular coupling (Tsurugizawa et al., 2010 and 2016), which potentially impacts the relationship between neuronal activation and vascular response. In particular, light sedation alters the BOLD response to physiological stimuli and thus affects the validity of task-based fMRI (Tsurugizawa et al., 2013a). Anesthesia clearly depresses consciousness, even if residual brain function is comparable to an awake state. In addition, it is impossible to perform cognitive tasks under anesthesia. In summary, fMRI under anesthesia, even with light anesthesia, is not comparable to fMRI in a conscious state, and thus rodent fMRI performed in an anesthetized state considerably narrows opportunities to comprehensively characterize brain function and connectivity. Hence, we developed an awake fMRI system for mice that does not necessitate anesthesia, thus enabling mouse fMRI experiments that are analogous to human fMRI.
Previous studies, including our own, have developed fMRI systems and protocols to acquire fMRI in awake mice. These have been used to investigate responses to fear conditioned stimulation (Harris et al., 2015) and optogenetics (Desai et al., 2011) and to investigate resting state functional connectivity (Bergmann et al., 2016; Yoshida et al., 2016; Madularu et al., 2017). Key limitations of the protocols used in these studies include the use of restraint tubes, absence of earplugs to reduce scanner noise (Mowery et al., 2019; Kurioka et al., 2020) and unclear animal handling and acclimation methods. We first developed an awake-mouse fMRI system to investigate the intragastric stimulation of capsaicin (Tsurugizawa et al., 2013b). More recently, we enhanced the system with the addition of a head fixation apparatus and acclimation training. The new system was used to investigate brain function in mice with a chromosome duplication (15q dup) resulting in abnormal behavior resembling ASD symptoms (Nakatani et al., 2009; Tsurugizawa et al., 2020b).
In this report, we describe our fMRI system and protocol for acquiring fMRI in awake mice, including cranioplastic surgery, acclimation training and fMRI acquisition. The protocol enables routine resting-state and task-fMRI mouse studies, where anesthetization of animals introduces a major confound.
Materials and Reagents
Small gauze pad
C57BL6J mice (8-15 weeks)
Super-Bond C & B (Sun medical)
GC UNIFAST Trad (GC Dental Products Corp., Aichi, Japan)
Male (VRSP6) and female (VRF6) Plastic Leur Fitting (Nordson Medical, US)
Earplugs for humans, made of Polyurethane (3M Company, Minnesota)
Equipment
A 4.7T horizontal MRI Avance IIII system (Bruker, Germany)
Mouse head positioner (custom made)
Dedicated mouse volume coil (30 mm diameter) to transmit/receive the radio frequency signal, combined with semicircular acrylic transparent plastic pipe for mouse bed (Takashima Seisakujo Co., Ltd)
Respiration/heart rate monitor system (Model 1025 , SA Instruments, Stony Brook, NY, USA)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Tsurugizawa, T., Tamada, K., Debacker, C., Zalesky, A. and Takumi, T. (2021). Cranioplastic Surgery and Acclimation Training for Awake Mouse fMRI. Bio-protocol 11(7): e3972. DOI: 10.21769/BioProtoc.3972.
- Tsurugizawa, T., Tamada, K., Ono, N., Karakawa, S., Kodama, Y., Debacker, C., Hata, J., Okano, H., Kitamura, A., Zalesky, A. and Takumi, T. (2020b). Awake functional MRI detects neural circuit dysfunction in a mouse model of autism. Sci Adv 6(6): eaav4520.
分类
神经科学 > 基础技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link