- EN - English
- CN - 中文
FRET-based Microscopy Assay to Measure Activity of Membrane Amino Acid Transporters with Single-transporter Resolution
用荧光共振能量转移的显微技术测定单转运体膜氨基酸转运体的活性
发布: 2021年04月05日第11卷第7期 DOI: 10.21769/BioProtoc.3970 浏览次数: 6752
评审: Pooja VermaRenuka KudvaAnonymous reviewer(s)
Abstract
Secondary active transporters reside in cell membranes transporting polar solutes like amino acids against steep concentration gradients, using electrochemical gradients of ions as energy sources. Commonly, ensemble-based measurements of radiolabeled substrate uptakes or transport currents inform on kinetic parameters of transporters. Here we describe a fluorescence-based functional assay for glutamate and aspartate transporters that provides single-transporter, single-transport cycle resolution using an archaeal elevator-type sodium and aspartate symporter GltPh as a model system. We prepare proteo-liposomes containing reconstituted purified GltPh transporters and an encapsulated periplasmic glutamate/aspartate-binding protein, PEB1a, labeled with donor and acceptor fluorophores. We then surface-immobilize the proteo-liposomes and measure transport-dependent Fluorescence Resonance Energy Transfer (FRET) efficiency changes over time using single-molecule Total Internal Reflection Fluorescence (TIRF) microscopy. The assay provides a 10-100 fold increase in temporal resolution compared to radioligand uptake assays. It also allows kinetic characterization of different transport cycle steps and discerns kinetic heterogeneities within the transporter population.
Keywords: Single-molecule FRET (单分子荧光共振能量转移)Background
Membrane resident secondary active transporters or solute carriers (SLC) mediate cellular uptake of amino acids, hormones, neurotransmitters, vitamins, and drugs, among other solutes. They couple concentrative substrate uptake to energetically favorable dissipation of electrochemical gradients of ions maintained primarily through the work of Na+/K+ ATPases (Lingrel and Kuntzweiler, 1994). The structural mechanisms of many secondary active transporters have come to light over the last decades (Grewer and Rauen, 2005; Shi, 2013; Vandenberg and Ryan, 2013; Drew and Boudker, 2016).
Measuring radioligand uptake into cells, membrane vesicles, or proteoliposomes is a traditional means to assay transport (Nimigean, 2006; Geertsma et al., 2008; Volpe, 2016). The cells and membrane vesicles provide the most native environment. By contrast, proteoliposomes allow precise control over compositions of membranes and luminal solutions. Broadly, purified transporters are reconstituted into liposomes with internal and external buffers providing the necessary ionic gradients. Gradient-dependent accumulation of the radiolabeled substrates in proteoliposomes is then measured. Specifically, a radiolabeled substrate is added to the external buffer, aliquots are taken at different time points and filtered. Finally, radioactivity trapped in the filters within the vesicles is measured. Initial rates of transport are measured as functions of substrate concentrations, lipid compositions, or temperature. These ensemble-based measurements provide mean uptake rates averaged over the population of transporters and over time. A shortcoming of the method relies on estimating what fraction of the transporters is reconstituted in an active form. Thus, accurate turnover times may be challenging to determine. Furthermore, the manual pipetting of aliquots limits the temporal resolution to 5 s or more, as reported in studies with various channels and transporters, including primary and secondary solute transporters and potassium channels (Knol et al., 1996; Heginbotham et al., 1998; Jung et al., 1998; van der Heide and Poolman, 2000; Enkvetchakul et al., 2004; Borths et al., 2005; Yamashita et al., 2005; Boudker et al., 2007).
We describe a single-molecule Fluorescence Resonance Energy Transfer (smFRET)-based uptake assay of liposome-reconstituted purified transporters (Figure 1). The current embodiment of this assay provides a temporal resolution of approximately 50 ms, a 100-fold increase compared to traditional radioligand uptake assays. The number and orientation of transporters in vesicles can be controlled. The assay detects the time it takes for each transporter to transport the first substrate molecule (during this time, the protein binds, translocates and releases the substrate into the vesicle lumen) and allows estimation of the mean turnover time of each transporter. A single measurement follows the activity of ca. 100-500 individual transporters simultaneously. The need for radioactive materials, which might be difficult and expensive to procure and are harmful to researchers and the environment, is eliminated.
We recently used this assay to study archaeal sodium and aspartate symporter, GltPh (Ciftci et al., 2020). We engineered a glutamate/aspartate FRET sensor based on a periplasmic glutamate/aspartate binding protein PEB1a by introducing two cysteine mutations for labeling with donor and acceptor fluorophores (ccPEB1a). The FRET efficiency of the labeled ccPEB1a increases as it binds an amino acid. We further mutated ccPEB1a to tune its aspartate affinity to allow tracking single- or up to 5-30 rounds of transport. GltPh containing a single cysteine mutation on the transporter's extracellular side was biotinylated and reconstituted into liposomes at 1:1,000 protein to lipid ratio (PLR) to enrich the population of proteoliposomes containing one transporter (Figure 1A). ccPEB1a was encapsulated by freeze-thaw cycles, and proteoliposomes were extruded through 0.1 µm filters, immobilized in smFRET imaging chambers, equipped with a rapid perfusion system, and imaged using TIRF microscopy (Figure 1B). The first half-cycle times for each transporter are determined manually from the onset of the FRET efficiency increase in a single-molecule trajectory following the substrate injection into the imaging chamber. The consequent continuous increase in FRET efficiency is fitted to a time-dependent aspartate binding equation (Ciftci et al., 2020) to obtain mean transporter turnover times (Figure 1C).
The assay was inspired by and, in part, based on a single-molecule assay developed for a neutral amino acid transporter MhsT utilizing periplasmic leucine, isoleucine, valine-binding protein LIV-BP (Fitzgerald et al., 2019). We expect analogous single-molecule transport assays to be developed for various other transporters, taking advantage of the natural diversity of periplasmic binding proteins (Berntsson et al., 2010) or other types of sensor proteins (Deuschle et al., 2005; Hou et al., 2011; Chen et al., 2013; Liu et al., 2015).
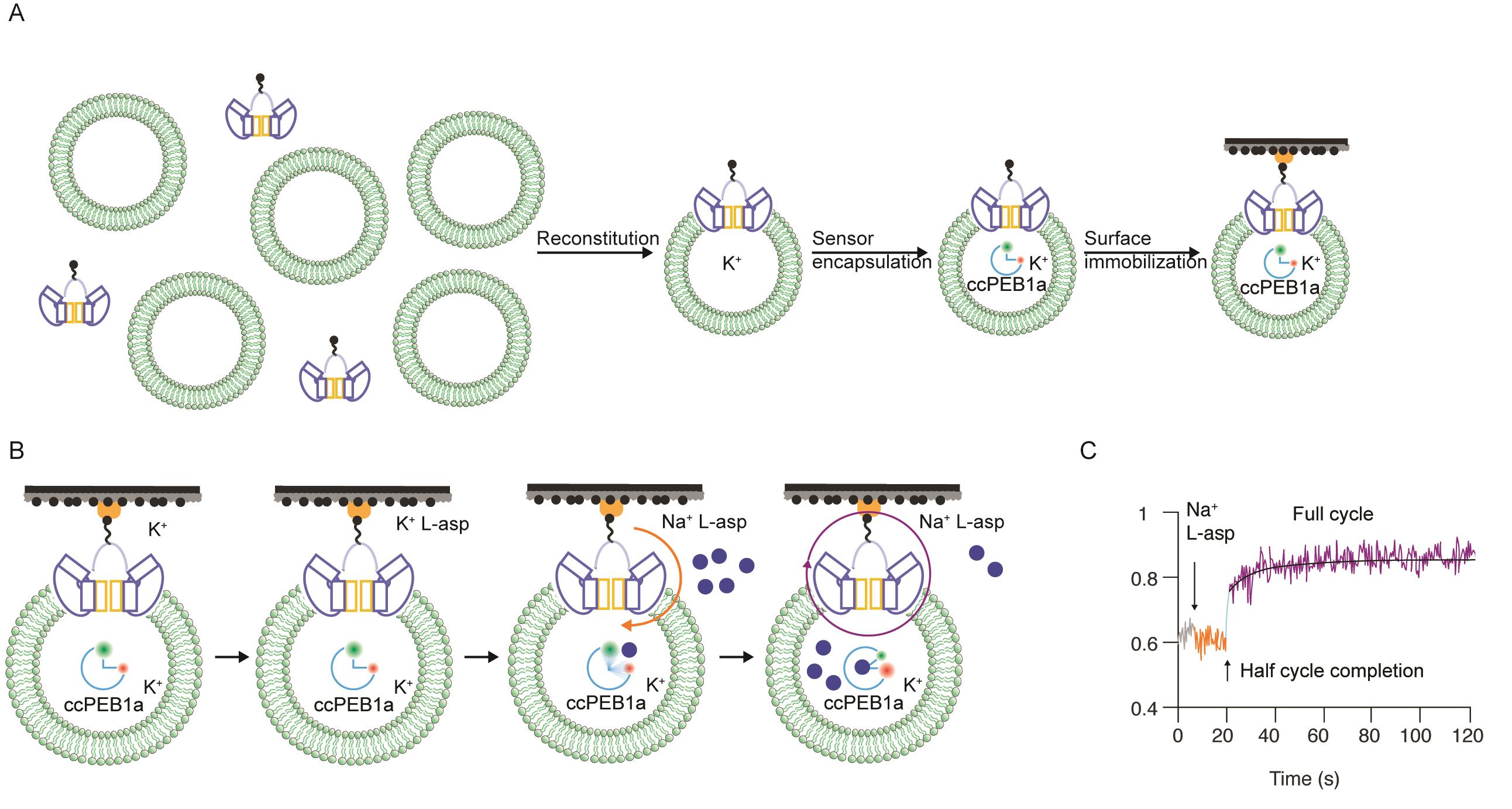
Figure 1. Single-molecule transport assay. A. Sample preparation flowchart. Biotinylated transporters are reconstituted into liposomes, the FRET sensor encapsulated, and proteoliposomes are immobilized in imaging chambers. B. Proteoliposomes are first imaged in a Resting buffer (K+), then a Non-activating buffer (K+/L-Asp) to test leakage, followed by an Activating buffer (Na+/L-Asp, blue circles) to initiate transport. Orange and purple arrows indicate the first half-cycle and consecutive turnovers, respectively. C. A representative single-molecule FRET efficiency trajectory.
Materials and Reagents
50 ml Syringe (BD, catalog number: 309654 )
Amicon Ultra-15 Centrifugal filters 100K (EMD Millipore, catalog number: UFC910096 )
Amicon Ultra-4 Centrifugal filters 100K (EMD Millipore, catalog number: UFC810096 )
Amicon Ultra-15 Centrifugal filters 10K (EMD Millipore, catalog number: UFC901024 )
Amicon Ultra-4 Centrifugal filters 10K (EMD Millipore, catalog number: UFC801024 )
Whatman Nucleopore Track-Etched membrane, 0.4 µm, 19 mm (Sigma-Aldrich, catalog number: WHA800282 )
Whatman Nucleopore Track-Etched membrane, 0.1 µm, 19 mm (Sigma-Aldrich, catalog number: WHA800309 )
Tube revolver/rotator for 1.5 ml reaction tubes
General Long-Term Storage Cryogenic Tubes (NalgeneTM, Thermo Fisher Scientific, catalog number: 5000-0020 )
50 ml tubes (Fisher Scientific, catalog number: 1443222 )
Glycerol (Fisher Scientific, catalog number: G334 )
Liquid N2
Chloroform (Fisher Scientific, catalog number: 507517453 )
Triton X-100 (Sigma-Aldrich, catalog number: T9284 )
LB broth, miller (Fisher Bioreagents, catalog number: BP1426-2 )
Ampicillin, sodium salt (GoldBio, catalog number: A-301-100 )
Ni-NTA superflow (QIAGEN, catalog number: 30430 )
Thiol reactive dyes (LD555, LD655) (Lumidyne Technologies, catalog numbers: LD555-MAL , LD655-MAL )
Dimethyl sulfoxide (DMSO) (Fisher Bioreagents, catalog number: BP231-100 )
Aspartate-glutamate binding protein, PEB1a (UniProt ID: M9QLL2. Mutations for site-specific labeling: C18S/N73C/K149C; mutations to tune affinity: A64F, D102L, Y198F, R89L)
Escherichia coli (E. coli) Polar Lipid Extract (Avanti Polar Lipids, catalog number: 100600C-100mg )
Egg phosphatidylcholine (PC) (Avanti Polar Lipids, catalog number: 840051C-25mg )
Bio-beads SM2 absorbent media (Bio-Rad, catalog number: 152-3920 )
Protocatechuic acid (PCA) (Sigma-Aldrich, catalog number: 37580-25G-F )
Protocatechuate-3,4-dioxygenase (PCD) (Sigma-Aldrich, catalog number: P8279 )
L-arabinose (GoldBio, catalog number: A-300-1 )
HEPES (Fisher Bioreagents, catalog number: BP310-100 )
Sodium chloride (NaCl) (Fisher Scientific, catalog number: S271-3 )
Potassium chloride (KCl) (EMD Millipore, catalog number: PX1405-1 )
Calcium chloride dihydrate (Sigma-Aldrich, catalog number: 223506 )
Tris (2-carboxyethyl) phosphine hydrochloride, 98% (TCEP) (Alfa Aesar, catalog number: 40587-09 )
Ethylenediaminetetraacetic acid disodium salt, dihydrate 100% (EDTA) (Alfa Aesar, catalog number: C1201100-500A5 )
Lysozyme, egg white (GoldBio, catalog number: L-040-10 )
Phenylmethylsulfonyl fluoride (PMSF) (MP Biomedicals, catalog number: 195381 )
Sucrose, ultrapure, RNase Free (MP Biomedicals, catalog number: 04821713 )
L-Aspartic acid, monopotassium salt (Research Products International, catalog number: 1115-63-5 )
n-Dodecyl-β-D-maltoside (DDM) (Anagrade, Anatrace, catalog number: D310 25 GM )
Thrombin from bovine plasma (Sigma-Aldrich, catalog number: T4648 )
β-D-1-thiogalactopyranoside (IPTG) (GoldBio, catalog number: I2481C100 )
Bovine serum albumin (BSA) (Fraction V, OmniPur, catalog number: 2930 )
Streptavidin (Invitrogen, catalog number: S888 )
Thermo ScientificTM EZ-LinkTM Maleimide-PEG11-Biotin (Fisher Scientific, catalog number: PI21911 )
N-ethyl maleimide (Sigma-Aldrich, catalog number: E3876 )
QIAprep spin miniprep kit (50×) (QIAGEN, catalog number: 27104 )
T7 express lysY competent E. coli (High efficiency) (New England Biolabs, catalog number: C3010I )
Thermo ScientificTM FastDigest DpnI (Fisher Scientific, catalog number: FERFD1704 )
PfuUltra II Hotstart PCR master mix (Agilent Technologies, catalog number: 600852 )
LB agar plates, ampicillin-100 (Teknova, catalog number: L1004 )
Pet21(+) vector (Genscript)
Resuspension buffer (see Recipes)
Wash buffer (see Recipes)
Elution buffer (see Recipes)
Size Exclusion Chromatography (SEC) buffer (see Recipes)
cysGltPh SEC buffer (see Recipes)
Resting buffer (see Recipes)
Non-activating buffer (see Recipes)
Activating buffer (see Recipes)
T50 buffer (see Recipes)
cysGltPh Resuspension buffer (see Recipes)
Equipment
Spectrophotometer (Horiba Photon Technology PTI, Quantamaster 8000 )
NanoDrop 2000c (Fisher Scientific, catalog number: ND-2000C )
2,800 ml Fernbach-style culture flasks with baffles (Fisher Scientific, catalog number: 09-552-39 )
Glass beaker
Magnetic stir bars
Superdex 200 Increase 10/300 GL column (GE Healthcare, catalog number: 28-9909-44 )
AKTA pure chromatography system (GE Lifesciences)
Aldrich® single-neck round-bottom flask, 100 ml capacity, Joint: ST/NS 24/40 (Sigma-Aldrich, catalog number: Z414492 )
BUCHI RotavaporTM R-300 Rotary Evaporator with Controller and V-300 Pump
Dry-seal vacuum desiccator (Fisher Scientific, catalog number: 086427)
Avanti Mini Extruder kit (Avanti Polar Lipids, catalog number: 610000 )
Filter Support (Avanti Polar Lipids, catalog number: 610014 )
MAXQ 8000 Incubated/Refrigerated Stackable Shaker (Thermo Fisher Scientific)
Drummond Portable Pipet-Aid (Thomas Scientific, catalog number: 13-661-17E )
Pipetman Kit, P1000, P200, P20, P10 (Gilson, catalog number: F167370 )
Avanti JXN-26 Centrifuge (Beckman Coulter)
Avanti JXN-30 Centrifuge (Beckman Coulter)
Optima MAX-XP Tabletop Ultracentrifuge (Beckman Coulter)
Optima L-100XP Ultracentrifuge (Beckman Coulter)
J-LITE JLA-8.1000 Fixed-Angle Aluminum Rotor, 6 × 1,000 ml, 8,000 rpm, 15,970 × g (Beckman Coulter, catalog number: 363688 )
JA-20 Fixed-Angle Aluminum Rotor, 8 × 50 ml, 20,000 rpm, 48,400 × g (Beckman Coulter, catalog number: 334831 )
Type 45 Ti Fixed-Angle Titanium Rotor (Beckman Coulter, catalog number: 339160 )
TLA-100.3 Fixed-Angle Rotor (Beckman Coulter, catalog number: 349481 )
1 L (1,000 ml) Polycarbonate Bottle with Cap Assembly, 95 × 191 mm (Beckman Coulter, catalog number: A98812 )
70 ml, Polycarbonate Bottle Assembly, 38 × 102 mm (Beckman Coulter, catalog number: 355622 )
26.3 ml, Polycarbonate Bottle with Cap Assembly, 25 × 89 mm (Beckman Coulter, catalog number: 355618 )
50 ml, Polycarbonate Bottle with Cap Assembly, 29 × 104 mm (Beckman Coulter, catalog number: 357000 )
Avestin EmulsiFlex C3 cell disruptor (Avestin)
Dounce tissue grinder set
XK 16/20 column (Cytivalifesciences, catalog number: 28988937 )
SpectrumTM Spectra/PorTM 4 RC Dialysis Membrane Tubing 12,000 to 14,000 Dalton MWCO (Fisher Scientific, catalog number: 08-667D )
SpectrumTM Dialysis Tubing Closures: Spectra/PorTM Standard Type (Fisher Scientific, catalog number: 08-670-11A )
Software
SPARTAN (Scott Blanchard laboratory, https://www.scottcblanchardlab.com/software)
MATLAB (MathWorks, https://www.mathworks.com/products/matlab.html)
Prism (GraphPad Software, Inc., https://www.graphpad.com/)
Origin (OriginLab Corporation, Northampton, MA, USA, https://www.originlab.com/)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Ciftci, D., Huysmans, G. H. M., Wang, X., He, C., Terry, D., Zhou, Z., Fitzgerald, G., Blanchard, S. C. and Boudker, O. (2021). FRET-based Microscopy Assay to Measure Activity of Membrane Amino Acid Transporters with Single-transporter Resolution. Bio-protocol 11(7): e3970. DOI: 10.21769/BioProtoc.3970.
- Ciftci, D., Huysmans, G. H. M., Wang, X., He, C., Terry, D., Zhou, Z., Fitzgerald, G., Blanchard, S. C. and Boudker, O. (2020). Single-molecule transport kinetics of a glutamate transporter homolog shows static disorder. Sci Adv 6(22): eaaz1949.
分类
生物物理学 > 显微技术
生物化学 > 蛋白质 > 单分子活性 > 成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link