- EN - English
- CN - 中文
Multilayered Fabrication Assembly Technique to Engineer a Corneal Stromal Equivalent
用多层制造组装技术制做角膜基质等效物
发布: 2021年03月20日第11卷第6期 DOI: 10.21769/BioProtoc.3963 浏览次数: 3351
评审: Alessandro DidonnaWilliam C. W. ChenKarem A Court
Abstract
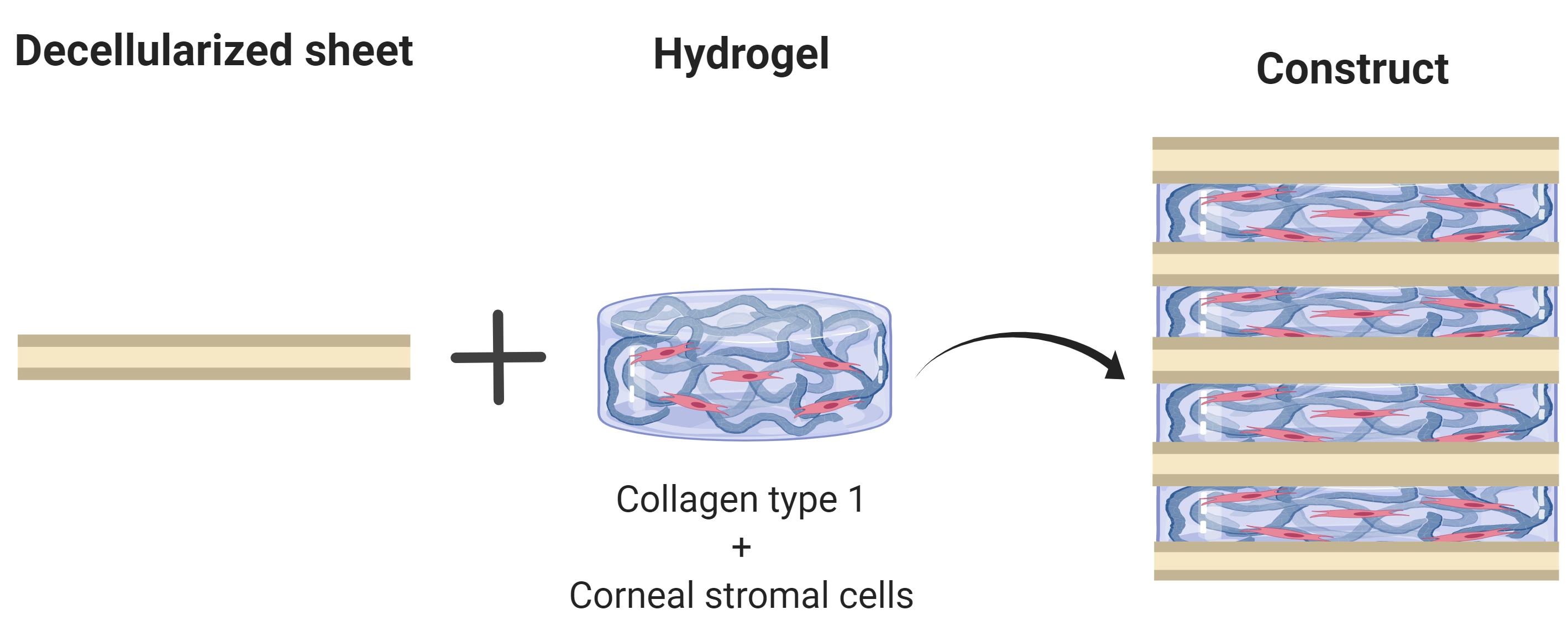
Tissue engineering has emerged as a strategy to combat the donor shortage of human corneas for transplantation. Synthetic corneal substitutes are currently unable to support the normal phenotype of human cells and so decellularized animal corneas have been deployed to more closely provide the topographical and biochemical cues to promote cell attachment and function. Although full thickness decellularized corneas can support corneal cells, the cells are slow to populate the scaffold and density declines from the surface. To avoid these problems, this protocol describes the stacking of alternate layers of decellularized porcine corneal sheets and cell-laden collagen hydrogel to produce a corneal construct. The sheets are obtained by cryosectioning porcine corneas, decellularizing them with detergents and nucleases and finally air drying for storage and ease of manufacture. Corneal stromal cells are then encapsulated in a collagen type I solution and cast between these sheets. This protocol presents a rapid method to ensure high cellularity throughout the construct using tissue-derived materials alone.
Graphic abstract:
Overview of main process to obtain corneal stromal equivalents
Background
Corneal blindness affects millions of people worldwide and treatment primarily relies upon the transplantation of human donor corneas (Gain et al., 2016). Since these donations are scarce, alternatives based on the tissue engineering of biomaterials are desirable. A wide range of strategies and materials are being developed to engineer corneal tissue, and one promising approach is the use of decellularized animal corneas (Fernández-Pérez and Ahearne, 2020) to provide a collagen-proteoglycan scaffold upon which the cells can grow. Decellularized porcine corneas have been approved as implant material in trials for the treatment of corneal ulcers, with encouraging clinical outcomes (Zhang et al., 2015, Zheng et al., 2019). However, after implantation, repopulation of the acellular implant by the migration of cells from the recipient can result in corneal haze or permanent scar tissue (Li et al., 2020). To avoid this problem, the decellularized cornea can be repopulated in vitro with cells prior to implantation. To achieve this and ensure high cell density, cells can be injected into the decellularized cornea, but this can damage the collagen architecture. Alternatively, cells seeded directly onto the scaffolds are slow to migrate into the tissue and their phenotype can be adversely affected. Our tactic is therefore to combine the decellularized corneal sheets with a high density of keratocytes in a collagen hydrogel to engineer the corneal stroma. In this protocol, we describe a way of fabricating corneal equivalents by stacking thin sheets of decellularized porcine corneas interleaved with cell-laden collagen hydrogels. These constructs have high cell viability, display characteristic stromal markers and are robust enough to be sutured in an ex vivo model (Fernández-Pérez et al., 2020). Whilst this protocol has been developed for the cornea, other researchers have reported a similar approach to produce tissue engineered cartilage (Gong et al., 2010).
Materials and Reagents
Pipette tips
12 and 24-well cell culture plates (Greiner, Cellstar, catalog numbers: 665180 and 662160 )
Screw Cap Urine Cup (Sarsted, catalog number: 75.9922.721 )
Plastic base mould (Simport, catalog number: HIS0221 )
Parafilm M (Amcor, catalog number: PM996 )
2 ml tube (Sarstedt, catalog number: 72.693.105 ) and 5 ml tube (Sarstedt, catalog number: 62.558.201 )
Sterile filter 0.2 μm (Corning, catalog number: 431219 )
Petri dish 100 mm diameter (Corning, catalog number: CLS43016 )
Cotton swab (autoclaved) (purchased from local supermarket)
Porcine eye globes (Local abattoir or butcher)
Note: For this protocol, age and strain of animals could not be controlled. This may also vary between countries.
Corneal stromal cells from human donor tissue (described in Fernández-Pérez and Ahearne, 2019b)
Corneal epithelial cells from human limbal biopsies, or an immortalized human corneal epithelial cell line (EverCyte, catalog number: hTCEpi )
Povidone-Iodine antiseptic 10% w/w (Ecolab, Videne, catalog number: HAS051P )
OCT embedding cryoembedding matrix (Thermo Fisher Scientific, catalog number: 12678646 )
Liquid nitrogen held in small insulated container
Phosphate buffered saline (PBS) without CaCl2 and MgCl2 (Sigma-Aldrich, catalog number: D8537 )
Decellularization solution. Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: L4390 ) and Triton X-100 (Sigma-Aldrich, catalog number: T8787 )
Magnesium chloride (Sigma-Aldrich, catalog number: M0250 )
Calcium chloride solution (Sigma-Aldrich, catalog number: 21115 )
DNase (Sigma-Aldrich, catalog number: DN25 )
RNase (Sigma-Aldrich, catalog number: R5000 )
Autoclaved de-ionized water
Antimicrobials, 2% v/v Penicillin-streptomycin (Gibco, catalog number: 15140122 ) and 0.25 μg/ml amphotericin B (Sigma-Aldrich, Merck, catalog number: A2942 ) in PBS or cell culture medium.
Teflon (polytetrafluoroethylene) cut into 12 mm diameter discs (Chemours, 2 mm thick sheet)
Rat tail collagen ~10 mg/ml (Corning, catalog number 354249 )
Dulbecco’s modified Eagle media (DMEM) low glucose (Hyclone, catalog number: 10529702 )
DMEM/F12 1:1 (Hyclone, catalog number: SH30023.01 )
Foetal bovine serum (FBS) (Gibco, catalog number: 10270106 )
Ascorbic acid (Sigma-Aldrich, catalog number: A8960 )
ITS 100× (Gibco, catalog number: 41400045 )
Keratinocyte growth medium (PromoCell, catalog number: C20011 )
Manual cell counter and hemocytometer (Blaubrand, catalog number: BR717805 )
10× PBS without CaCl2 and MgCl2 (Corning, catalog number: 46-013-CM )
Phenol Red, sodium salt (Sigma-Aldrich, catalog number: P5530 )
1 M NaOH (Honeywell, catalog number: CCS8045 )
Decellularization solution (see Recipes)
MgCl2 Buffer (see Recipes)
DNase & RNase solutions (see Recipes)
10× PBS with phenol red (see Recipes)
Stromal cell media (see Recipes)
Keratocytic media (see Recipes)
Equipment
Dissection and manipulation tools (Sterile forceps, scissors, scalpels including a 2 mm wide spatula bent to a L-shape (Witig, catalog number: WITG7.412.051 )
Biopsy punch 10 mm diameter (Acuderm, Acu-Punch, catalog number: P1050 )
Cryostat (Leica, model number: CM1850UV )
-80 °C freezer (Thermo Fisher, Forma Model 906 )
Orbital shaker (Witeg SHO-1D)
Stirring plate (Henry Troemner, VWR, Advanced series 4x4)
Centrifuge (Hettich, model: 320R )
37 °C oven (Memmert, UF55 )
Biosafety cabinet (Faster S.r.l., SafeFAST Classic, 212 )
Humidified CO2 incubator (Thermo Scientific, Forma Steri-Cycle, i790 )
Vortexer (Fisherbrand, Mini-E, catalog number: 15212985 )
Benchtop mini centrifuge (StarLab, catalog number: SLN2631-0007 )
Pipettes (Brand, Transferpette, catalog number: 705891 )
Cooling tray. Expanded Polystyrene container with melting ice or pre-cooled metallic beads (Lab Armor Beads)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Fernandez-Perez, J., Madden, P. W. and Ahearne, M. (2021). Multilayered Fabrication Assembly Technique to Engineer a Corneal Stromal Equivalent. Bio-protocol 11(6): e3963. DOI: 10.21769/BioProtoc.3963.
分类
生物工程 > 生物医学工程
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










