- EN - English
- CN - 中文
Characterize the Interaction of the DNA Helicase PriA with the Stalled DNA Replication Fork Using Atomic Force Microscopy
用原子力显微镜描述DNA解旋酶PriA与停止的DNA复制叉的相互作用
发布: 2021年03月05日第11卷第5期 DOI: 10.21769/BioProtoc.3940 浏览次数: 4850
评审: Alexander KotlyarLip Nam LOHAnonymous reviewer(s)
Abstract
In bacteria, the restart of stalled DNA replication forks requires the DNA helicase PriA. PriA can recognize and remodel abandoned DNA replication forks, unwind DNA in the 3'-to-5' direction, and facilitate the loading of the helicase DnaB onto the DNA to restart replication. ssDNA-binding protein (SSB) is typically present at the abandoned forks, protecting the ssDNA from nucleases. Research that is based on the assays for junction dissociation, surface plasmon resonance, single-molecule FRET, and x-ray crystal structure has revealed the helicase activity of PriA, the SSB-PriA interaction, and structural information of PriA helicase. Here, we used Atomic Force Microscopy (AFM) to visualize the interaction between PriA and DNA substrates with or without SSB in the absence of ATP to delineate the substrate recognition pattern of PriA before its ATP-catalyzed DNA-unwinding reaction. The protocol describes the steps to obtain high-resolution AFM images and the details of data analysis and presentation.
Keywords: Atomic force microscopy (原子力显微镜)Background
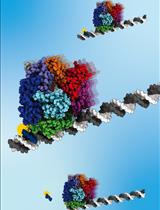
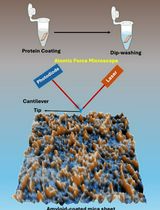
When DNA replication encounters roadblocks or breakage, it needs to be repaired and restarted afterward (Kogoma, 1997; Cox et al., 2000; McGlynn and Lloyd, 2002; Gabbai and Marians, 2010; Michel et al., 2018). In bacteria, the DNA helicase PriA mediates this process by recognizing the abandoned DNA replication fork, facilitating the reassembly of the replisome and loading of the helicase DnaB (Wickner and Hurwitz, 1975; Zavitz and Marians, 1992; Sandler and Marians, 2000; Michel et al., 2004; Windgassen et al., 2018b). Studies based on the assays for junction dissociation, surface plasmon resonance, and single-molecule FRET have elaborated on the preference of PriA to the fork DNA structures based on the helicase activity and the protein-protein interaction of PriA with other proteins (Zavitz and Marians, 1992; Cadman and McGlynn, 2004; Bhattacharyya et al., 2014; Yu et al., 2016). The x-ray crystal structure described the structural mechanism of how PriA recognizes and processes the branched DNA replication forks (Bhattacharyya et al., 2014; Windgassen et al., 2018a; Windgassen et al., 2019). In this work, we applied Atomic Force Microscopy (AFM) to visualize the PriA-DNA complex topographically. The protocol describes how to use AFM to characterize the complexes based on the analyses of AFM images.
Materials and Reagents
Amicon Ultra-0.5 ml centrifugal filters (Millipore-Sigma, UFC503008 , pore size: 30 kDa NMWCO)
Nonwoven cleanroom wipes: TX604 TechniCloth (TexWipe, catalog number: TX604 )
Petri dish (Fisher Scientific, catalog number: 08-757-100A )
Standard disposal cuvette (Perfector Scientific, catalog number: 9002 )
Distilled deionized H2O (DDI H2O)
pUC19 Vector (New England Biolabs, catalog number: N3041S )
PCR primers (IDT, custom order)
F364: 5’-GAGTTCTTGAAGTGGTGGCC-3’
R364: 5’-GGTAACTGTCAGACCAAGTTTACTC-3’
F480: 5’-GCGATTAAGTTGGGTAAC-3’
R480: 5’-GTTCTTTCCTGCGTTATC-3’
DreamTaq polymerase (Thermo Fisher Scientific, catalog number: EP0701 )
Deoxynucleotide (dNTP) Solution Mix (New England Biolabs, catalog number: N0447S )
PCR purification kit (Qiagen, catalog number: 28104 )
Restriction endonuclease: DdeI (New England Biolabs, catalog number: R0175S )
Restriction endonuclease: BspQI (New England Biolabs, catalog number: R0712S )
CutSmart® Buffer (New England Biolabs, catalog number: B7204S )
Oligonucleotide (IDT, custom order)
O30: 5’-TCATCTGCGTATTGGGCGCTCTTCCGCTTCCTATCT-3’
O31: 5’-TCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCATA-3’
O32: 5’-GCTTATGAGTGAGCTGATACCGCTCGCCGCAGCCGAACGACCTTGCGCAGCGAGTCAGTGAGATAGGAAGCGGAAGAGCGCCCAATACGCAGA-3’
O33: 5’-CACTGACTCGCTGCGCAAGGCTAACAGCATCACACACATTAACAATTCTAACATCTGGGTTTTCATTCTTTGGGTTTCACTTTCTCCAC-3’
O34: 5’-CTAACAGCATCACACACATTAACAATTCTAACATCTGGGTTTTCATTCTTTGGGTTTCACTTTCTCCACCACTGACTCGCTGCGCAAGG-3’
O36: 5’-TACGTGTAGGAATTATATTAAAGAGAAAGTGAAACCCAAAGAATGAAAAAGAAGATGTTAGAATTGTAAGCGGTATCAGCTCACTCATA-3’
O37: 5’-GCTTATGAGTGAGCTGATACCGC-3’
O42: 5’-TCATGACTCGCTGCGCAAGGCTAACAGCATCACACACATTAACAATTCTAACATCTGGG TTTTCATTCTTTGGGTTTCACTTTCTCCAC-3’
O43: 5’-CCTTGCGCAGCGAGTCA-3’
T4 Polynucleotide Kinase (New England Biolabs, catalog number: M0201S )
T4 DNA Ligase (Thermo Fisher Scientific, catalog number: 15224090 )
DL-Dithiothreitol (Sigma-Aldrich, catalog number: 43819-5G )
EDTA (Thermo Fisher Scientific, catalog number: 15576028 )
Tris base (Sigma-Aldrich, catalog number: 10708976001 )
Phenol:Chloroform:Isoamyl Alcohol 25:24:1, Saturated with 10 mM Tris, pH 8.0, 1 mM EDTA (Sigma-Aldrich, catalog number: P3803-100ML )
Isopropanol (Fisher Scientific, catalog number: A426P-4 )
Ethanol (Decon Labs, catalog number: 2701 )
Sodium acetate buffer solution, pH 5.2±0.1 (25 °C), 3 M, 0.2 μm filtered (Sigma-Aldrich, catalog number: S7899-100ML )
Acetic acid (ACROS Organics, catalog number: AC124040010 )
HCl (Sigma-Aldrich, catalog number: 258148-25ML )
Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M8266-100G )
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888-500G )
Muscovite Block Mica (AshevilleMica, catalog number: Grade-1 )
1-(3-Aminopropyl) silatrane (APS) [synthesized as described in ref. (Shlyakhtenko et al., 2013)]
TESPA-V2 AFM probe (Bruker AFM Probes, catalog number: TESPA-V2 )
Platinum coated calibration grid, 1 µm × 1 µm period (Bruker AFM Probes, catalog number: PG )
10× binding buffer (see Recipes)
Equipment
Aquamax Water Purification System (APS Water Services corporation)
PCR Thermal cycler (Eppendorf, catalog number: 5332-54318 )
Votexer (Glas-Col, catalog number: 099A PV6 )
HPLC (Shimadzu, catalog numbers: 228-34350-92 ; 228-35555-92 ; 228-39001-92 ; 228-39001-92 ; 228-39005-92 )
TSKgel DNA-STAT column: 4.6 mm I.D. × 10 cm, 5 μm (Tosoh, catalog number: 821962 )
ND-1000 NanoDrop Spectrophotometers (ThermoFisher Scientific, listing number: E112352 )
MultiMode 8, Nanoscope V system (Bruker, model number: MMAFM-2 )
Software
FemtoScan Online (Advanced Technologies Center, Moscow, Russia, http://www.nanoscopy.net/en/Femtoscan-V.shtm)
Origin (OriginLab Corporation, Northampton, MA, USA, https://www.originlab.com/)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Wang, Y., Sun, Z., Bianco, P. R. and Lyubchenko, Y. L. (2021). Characterize the Interaction of the DNA Helicase PriA with the Stalled DNA Replication Fork Using Atomic Force Microscopy. Bio-protocol 11(5): e3940. DOI: 10.21769/BioProtoc.3940.
- Wang, Y., Sun, Z., Bianco, P.R. and Lyubchenko, Y.L. (2020). Atomic force microscopy–based characterization of the interaction of PriA helicase with stalled DNA replication forks. J Biol Chem 295(18): 6043-6052.
分类
生物物理学 > 显微技术 > 原子力显微镜
微生物学 > 微生物生物化学 > DNA
分子生物学 > DNA > DNA-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link