- EN - English
- CN - 中文
Extraction and Quantification of Sphingolipids from Hemiptera Insects by Ultra-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry
超高效液相色谱-串联质谱联用法提取和定量半翅目昆虫鞘脂质
发布: 2021年02月20日第11卷第4期 DOI: 10.21769/BioProtoc.3923 浏览次数: 5229
评审: Agnieszka ZienkiewiczMarc-Antoine SaniIstvan Stadler
Abstract
Sphingolipids are major structural components of endomembranes and have also been described as an intracellular second messenger involved in various biological functions in all eukaryotes and a few prokaryotes. Ceramides (Cer), the central molecules of sphingolipids, have been depicted in cell growth arrest, cell differentiation, and apoptosis. With the development of lipidomics, the identification of ceramides has been analyzed in many species, mostly in model insects. However, there is still a lack of research in non-model organisms. Here we describe a relatively simple and sensitive method for the extraction, identification, and quantification of ceramides in Hemiptera Insects (brown planthooper), followed by Ultra-Performance Liquid Chromatography coupled to tandem mass spectrometry (UPLC-MS/MS). C18 is used as the separation column for quantitative detection and analysis on the triple quadruple liquid mass spectrometer. In this protocol, the standard curve method is adopted to confirm the more accurate quantification of ceramides based on the optional detection conditions.
Keywords: Hemipetera Insects (半翅目昆虫)Background
Sphingolipids are the second largest group of membrane lipids in living organisms and play an important role in many aspects of cell structure, metabolism, and regulation (Lahiri and Futerman, 2007). At first, it was thought that sphingolipids were a complex family of structurally related molecules, but more and more studies have shown that sphingolipids are involved in numerous cellular processes (Mao and Obeid, 2008). Ceramides (Cer) are essential bioactive lipids implicated in various cell biological processes ranging from cell growth regulation to cell death and senescence (Futerman and Hannun, 2004; Hannun and Obeid, 2008) through influencing of multiple signaling pathways. Although the physiological roles of ceramides are widely reported, few studies have described the extraction, identification, and quantification, thus, analysis of ceramides has gained significant interest in investigating the physiological functions of sphingolipid metabolism in Hemiptera Insects.
Currently, various methods have been described for this purpose, such as Diacylglycerol (DAG) Kinase assay (Preiss et al., 1987), Thin-layer chromatography (TLC) (Gorska et al., 2002), Gas chromatography mass spectrum (GC-MS) (Tserng et al., 2003), High-performance liquid chromatography (HPLC) (Yano et al., 1998; Dobrzyn and Gorski, 2002). In the beginning, DAG kinase assay was commonly used for Cer quantitation, but the specificity has been questioned (Watts et al.,1997). Thin layer chromatography was the method of choice, but the resolution, sensibility, and separation were limited, resulting in inefficient separation of similar molecules (Bielawski et al., 2010). Despite the high sensitivity of chromatographic analysis, this method had some limitations, such as the need for standard substances and derivatization (Dobrzyn et al., 2004). High-performance liquid chromatography (HPLC) was introduced to obtain ceramides separation with higher resolution, but complex samples like tissue extracts, therefore, produced many unspecific signals that did not provide any information concerning the metabolism of molecular species by HPLC (Yano et al., 1998; Bode and Graler, 2012).
Given the ultra performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS), it is more efficient, rapid, and sensitive, improving the separation condition of extremely complex samples and reducing matrix interference (Cutignano et al., 2010). Therefore, the current choice method is the analysis of ceramides by Ultra Performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS). This protocol provides a relatively rapid and reproducible method. Moreover, this method can be used to profile ceramides from the extraction of the plant sample. It has evolved as the method of choice to detect sphingolipids metabolites due to its high sensitivity and superior specificity. The content of ceramide is determined and a quantitative system of sphingolipids in Hemiptera Insects is established, which lay a foundation for understanding the metabolic process and elucidating the biological function of each component. This method was described and used successfully to extract other sphingolipids in previously published studies (Bielawski et al., 2010; Shi et al., 2018 and 2019).
Materials and Reagents
1.5 ml Eppendorf tubes (Axygen, catalog number: MCT-150-C )
1.0 mm Ceramic Beads (Nalgene, catalog number: 150010C )
2 ml Micro tube (Sarstedt, catalog number: 72.609 )
Pipette tips (Axygen, catalog numbers: T-300 , T-200-Y , T-1000-B )
Glass Centrifuge Tubes (VWR International, catalog number: 734-4240 )
Nitrogen gas (> 99% Purity) (any brand will suffice)
Isopropyl alcohol (Sangon Biotech, catalog number: A503069 )
Ethyl acetate (Sangon Biotech, catalog number: A507048 )
Liquid nitrogen (any brand will suffice)
HPLC-grade methanol (Sigma-Aldrich, catalog number: 34806 )
Formic acid (Sangon Biotech, catalog number: A503066 )
MilliQ Water (Millipore, catalog number: Direct-Q3 )
Standards (see Table 1)
Note: All Ceramides standards list is shown in Table 1.
Solvent extraction solution A (see Recipes)
Solvent extraction solution B (see Recipes)
Mobile phase A (see Recipes)
Mobile phase B (see Recipes)
Internal standard (Avanti company) (see Recipes)
Table 1. Example of ceramides standards list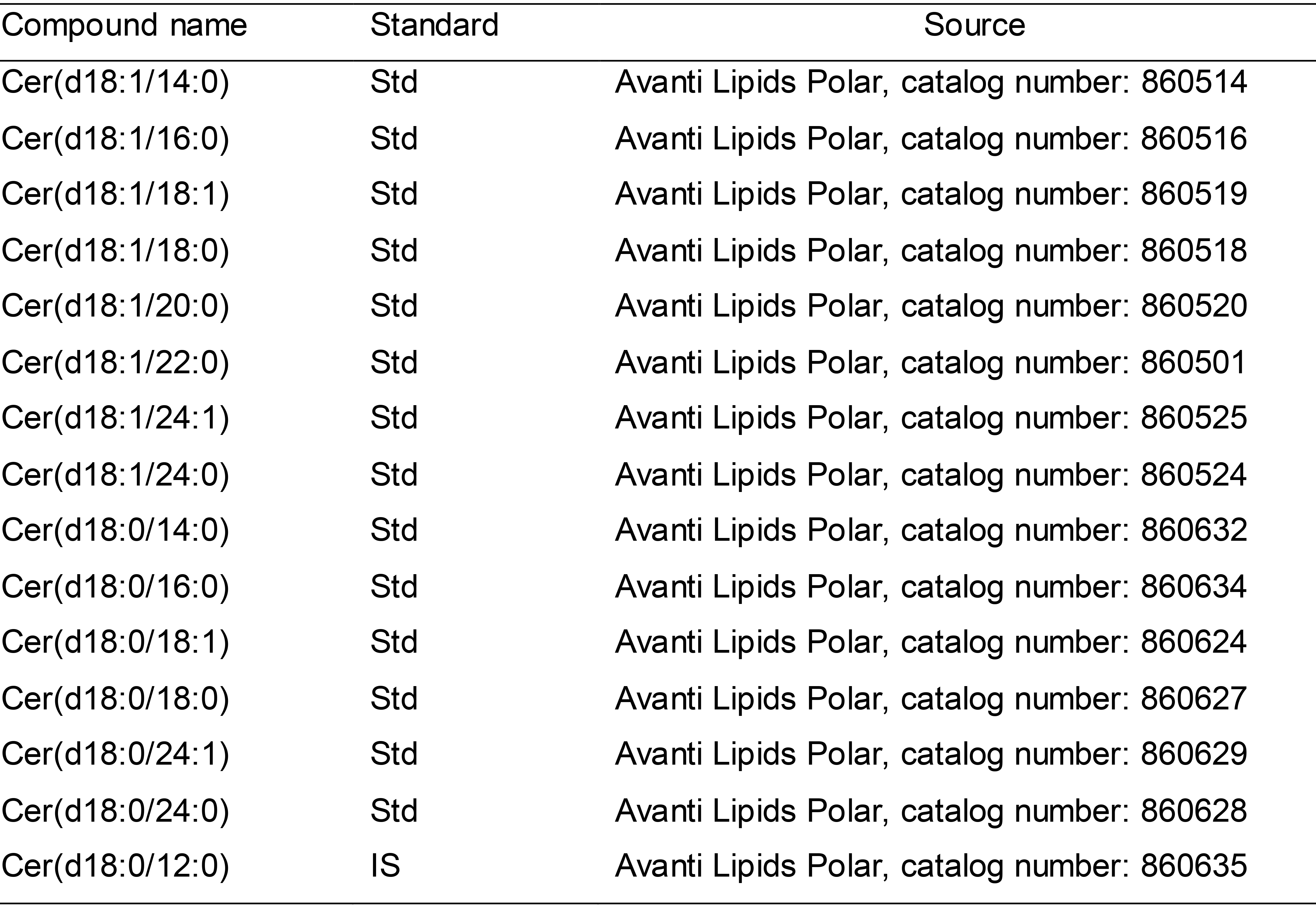
Std: Standard; IS: Internal standard
Equipment
Nitrogen evaporator N-EVAP (Organomation, model: HGC-24A )
Centrifuge (Eppendorf, model: 5430R )
Autoclave (SANYO, model: MLS-3780 )
UHPLC-Q-TOF-MS/MS system (AB SCIEX, Framingham, MA, USA)
UHPLC column (Zorbax sb-C8, 2.1 × 150 mm, 3.5 μm; Agilent, Palo Alto, CA, USA)
Ivory PTFE/red silicone rubber septa (Agilent Technologies, catalog number: 5182-0731 )
2 ml amber screw vial with patch USP 1 expansion (HAMAG Technologies, catalog number: HM-0716H )
Blue open-topped polypropylene cap and white PTFE/red Scilicone septa (HAMAG Technologies, catalog number: HM-0722 )
250 μl clear glass pulled conical-bottom (HAMAG Technologies, catalog number: HM-2085 )
Analytical balance (METTLER TOLEDO, model: XS105 )
Tissue homogenizer (MP Biomedicals, USA, FastPrep-24 )
Oven (Bluepard, model: BPG-9040A )
Vortexer (Germany, IKA, model: vortex 2 )
-80 °C freezer
Software
PeakView (AB Sciex)
Excel software (Microsoft office 2010)
Data Processing software (DPS)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Wang, N., Shi, X., Zhang, C., Zhou, W. and ZHU, Z. (2021). Extraction and Quantification of Sphingolipids from Hemiptera Insects by Ultra-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Bio-protocol 11(4): e3923. DOI: 10.21769/BioProtoc.3923.
分类
生物化学 > 脂质 > 膜脂
发育生物学 > 细胞生长和命运决定 > 分化
细胞生物学 > 细胞新陈代谢 > 脂质
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link