- EN - English
- CN - 中文
Resolving Structural Changes of Photoreceptors in Living Escherichia coli via In-cell Infrared Difference Spectroscopy
利用细胞内红外差谱技术分析活大肠杆菌光感受器的结构变化
发布: 2021年02月05日第11卷第3期 DOI: 10.21769/BioProtoc.3909 浏览次数: 4534
评审: Ali Asghar KermaniShailesh KumarAnonymous reviewer(s)
Abstract
Several in-cell spectroscopic techniques have been developed recently to investigate the structure and mechanism of proteins in their native environment. Conditions in vivo differ dramatically from those selected for in vitro experiments. Accordingly, the cellular environment can affect the protein mechanism for example by molecular crowding or binding of small molecules. Fourier transform infrared (FTIR) difference spectroscopy is a well-suited method to study the light-induced structural responses of photoreceptors including changes in cofactor, side chains and secondary structure. Here, we describe a protocol to study the response of cofactor and protein in living E. coli cells via in-cell infrared difference (ICIRD) spectroscopy using the attenuated total reflection (ATR) configuration. Proteins are overexpressed in E. coli, the cells are transferred into saline solution and the copy number per cell is determined using fluorescence spectroscopy. The suspension is centrifuged and the concentrated cells transferred onto the ATR cell inside the FTIR spectrometer. The thermostatted cell is sealed and illuminated from the top with an LED. Intensity spectra are recorded before and after illumination to generate the difference spectrum of the receptor inside the living cell. With ICIRD spectroscopy, structural changes of soluble photoreceptors are resolved in a near-native environment. The approach works in H2O at ambient conditions, is label free, without any limitations in protein size and does not require any purification step.
Graphic abstract:
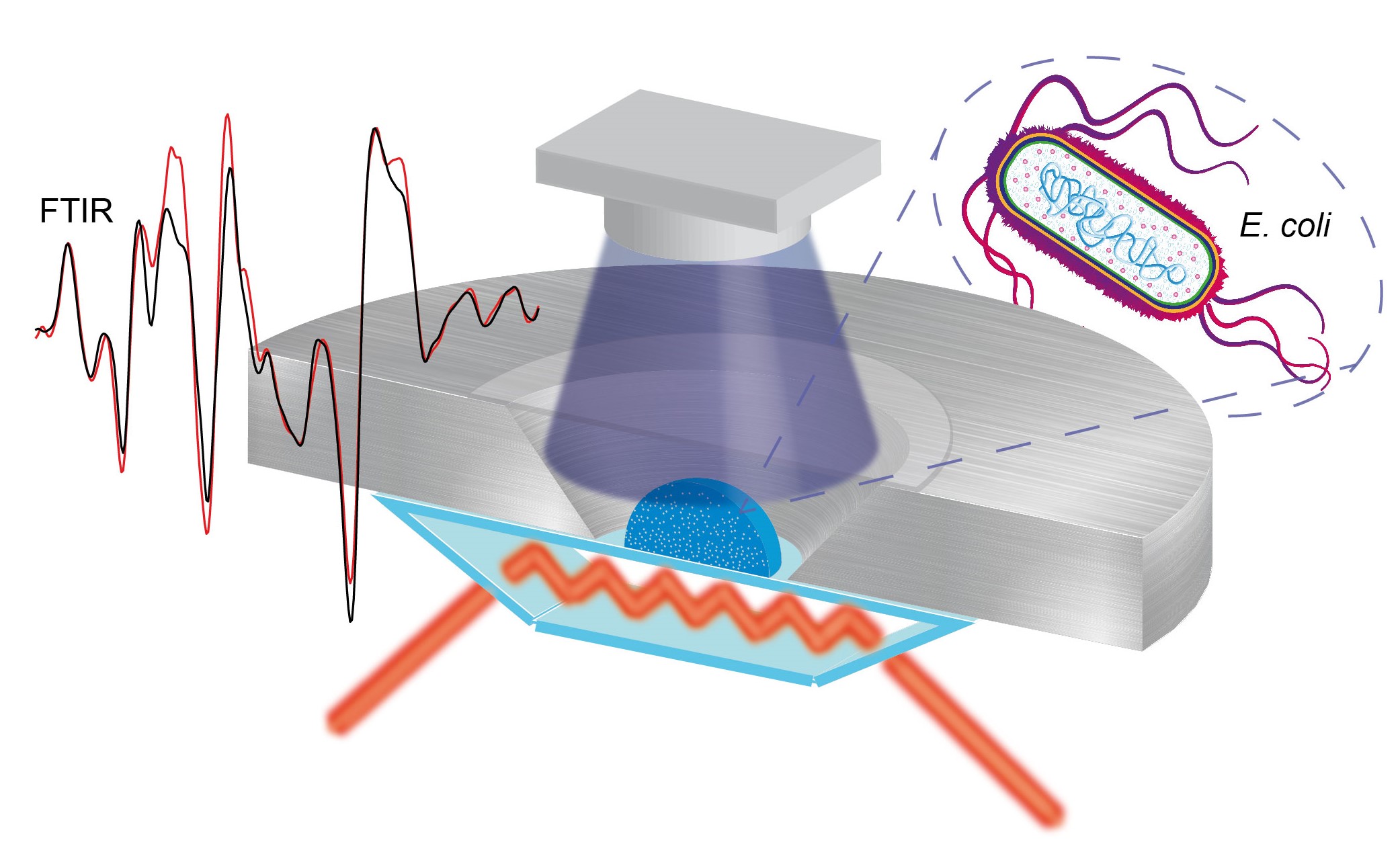
In-cell infrared difference spectroscopy on photoreceptors in living E. coli using attenuated total reflection.
Background
Photoreceptors play an essential role in light sensing and light adaption in organisms varying from bacteria, fungi, algae and plants to animals (Möglich et al., 2010; Gomelsky and Hoff, 2011; Losi and Gärtner, 2012; Ernst et al., 2014; Jaubert et al., 2017). The photoreceptors are usually studied in a well-defined buffer in vitro, but deviations between in vitro and in vivo conditions might change the mechanisms of the receptors. Methods to investigate the light-induced response of photoreceptors in the cellular environment have been developed including UV-visible, fluorescence and EPR spectroscopy (Butler et al., 1963; Hennig et al., 1999; Banerjee et al., 2007; Bouly et al., 2007). These important studies address the investigation of the cofactor and its photoreaction, but provide only limited information about the protein moiety.
FTIR difference spectroscopy is a well-established method to reveal the structural response to light of cofactor and protein moiety in vitro (Mäntele, 1993; Barth and Zscherp, 2002; Garczarek and Gerwert, 2006; Kottke et al., 2017). IR difference spectroscopy was applied to study the response of the insoluble membrane photoreceptor rhodopsin to light in single animal rod cells using synchrotron radiation (Quaroni et al., 2011). Rod cells were chosen, because they contain a high native concentration of rhodopsin in the membrane. Soluble photoreceptors include many different receptor families such as the light, oxygen and voltage (LOV) proteins, cryptochromes, blue light using flavin proteins, phytochromes or photoactive yellow proteins, and are usually present at low concentrations in the cytosol of the native host. We recently developed the ICIRD spectroscopy in the transmission mode and the ATR configuration to study the light-induced structural response of the soluble LOV domains in tandem with the effector domain in living E. coli cells at only 300,000 copies per cell using an FTIR spectrometer (Goett-Zink et al., 2020). ICIRD spectroscopy might be extended to study members of other photoreceptor families and the evolution of structural changes by combination with a time-resolved approach. Moreover, other receptors and enzymes might be investigated in living cells by a combination with optogenetic tools.
Materials and Reagents
Disposable cuvettes 2.5 ml macro PMMA (Brand, catalog number: 7591 05 )
Centrifuge tubes 50 ml (VWR, catalog number: 525-1099 )
Eppendorf tubes 2 ml (Merck, catalog number: BR780546-500EA )
Pipette tips 0.5-5 ml (Roth, catalog number: HL73.1 )
Pipette tips 200 µl (Th. Geyer, catalog number: 7695843 )
Fluorescence cuvette for magnetic stirrers, 10 × 4 mm (Hellma, catalog number: 119004F-10-40 )
Round coverslip Ø 15 mm (Roth, catalog number: P232.1 )
BL21 (DE3) competent cells (Thermo Fisher Scientific, catalog number: EC0114 )
Milli-Q water
Agar-Agar, BioScience (Roth, catalog number: 6494.1 )
Kanamycin monosulfate (MP Biomedicals, catalog number: 11476412 )
Sodium chloride (Roth, catalog number: 9265.1 )
Potassium chloride (Roth, catalog number: 6781.1 )
di-Potassium hydrogen phosphate (VWR, catalog number: 71003-452 )
Potassium di-hydrogen phosphate (VWR, catalog number: BDH9268-500G )
Tryptone (MP Biomedicals, catalog number: 1010817 )
Yeast extract powder (MP Biomedicals, catalog number: 103303 )
Isopropyl-β-d-thiogalactopyranoside (MP Biomedicals, catalog number: 102101 )
Liquid nitrogen (Linde)
Silicone-free laboratory grease (VWR, catalog number: DECO514215.00-CA15 )
Double yeast tryptone (DYT) medium (see Recipes)
Saline solution (see Recipes)
Phosphate buffer 50 mM, pH 8.0, NaCl 300 mM (see Recipes)
Equipment
Pipette 2-20 µl (Brand, catalog number: 705870 )
Pipette 20-200 µl (Brand, catalog number: 705882 )
Pipette 0.5-5 ml (Brand, catalog number: 705882 )
Erlenmeyer flask 500 ml (Roth, catalog number: C139.1 )
Erlenmeyer flask with chicanes (Roth, catalog number: C139.1 ) (chicanes home-built)
Magnetic stir bar Ø 3-4 mm, length 6-7 mm (Hellma, catalog number: 323-300-VE10 )
Incubator, Multitron Standard (INFORS HT)
Refrigerated centrifuge, Multifuge 3 L-R (Thermo Fisher Scientific, catalog number: 10563772 )
Centrifuge, Mikro 200 (Hettich, catalog number: 2405 )
Cell density meter, Ultrospec 10 (Biochrom, catalog number: 80-2116-300 )
Fluorescence spectrometer, Spectrofluorometer FP-8300 (Jasco)
Absorptive neutral density filter, Schott NG 4, Ø 25 mm, 3 mm thickness, 2.6% (Edmund Optics, catalog number: 14-091 )
FTIR spectrometer, IFS 66v or IFS 66/s (Bruker)
ATR unit for FTIR spectrometer, MIRacle Base Optics/Platform Assembly (Pike Technologies, catalog number: 025-1850 )
Nine-reflection diamond/ZnSe performance crystal plate, with trough (Pike Technologies, catalog number: 025-2218 )
IR bandpass filter, 5% cut-on at 5.35 µm, 5% cut-off at 9 µm, Ø 25 mm (Laser Components, catalog number: 3007570 )
Dry air generator, dew point -70 °C (Balston)
Water cooling unit for crystal plate (home-built), alternative: digital temperature control module (Pike Technologies, catalog number: 076-1220 and 076-1420 )
Refrigerated circulator, digital temperature controller (PolyScience)
LED 451 nm, 60 mW·cm-2 at the sample, Luxeon Star/O (Phillips Lumileds) (might be adapted for photoreceptors other than LOV)
LED holder (home-built)
Light shaping diffuser, 5°, diffuser kit, circular light shaping, narrow, Ø 25.4 mm (Newport, catalog number: 10DKIT-C1 )
Software
OPUS 7.5 (Bruker, www.bruker.com)
OriginPRO 2020 (OriginLab, www.originlab.com)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Goett-Zink, L., Klocke, J. L. and Kottke, T. (2021). Resolving Structural Changes of Photoreceptors in Living Escherichia coli via In-cell Infrared Difference Spectroscopy. Bio-protocol 11(3): e3909. DOI: 10.21769/BioProtoc.3909.
- Goett-Zink, L., Klocke, J. L., Bögeholz, L. A. K. and Kottke, T. (2020). In-cell infrared difference spectroscopy of LOV photoreceptors reveals structural responses to light altered in living cells. J Biol Chem 295(33): 11729-11741.
分类
生物物理学 > 红外光谱
微生物学 > 体内实验模型 > 细菌
生物化学 > 蛋白质 > 结构
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link