- EN - English
- CN - 中文
Carboxyfluorescein Dye Uptake to Measure Connexin-mediated Hemichannel Activity in Cultured Cells
用羧荧光素染料上染率测定培养细胞中连接蛋白介导的半通道活性
发布: 2021年02月05日第11卷第3期 DOI: 10.21769/BioProtoc.3901 浏览次数: 5315
评审: Xiaoyi ZhengYing ShiAnonymous reviewer(s)

相关实验方案
采用 Davidson 固定液和黑色素漂白法优化小鼠眼组织切片的免疫组化染色
Anne Nathalie Longakit [...] Catherine D. Van Raamsdonk
2025年11月20日 1562 阅读
Abstract
Connexins are membrane bound proteins that facilitate direct and local paracrine mediated cell-to-cell communication through their ability to oligomerise into hexameric hemichannels. When neighbouring channels align, they form gap-junctions that provide a direct route for information transfer between cells. In contrast to intact gap junctions, which typically open under physiological conditions, undocked hemichannels have a low open probability and mainly open in response to injury. Hemichannels permit the release of small molecules and ions (approximately 1kDa) into the local intercellular environment, and excessive expression/activity has been linked to a number of disease conditions. Carboxyfluorescein dye uptake measures functional expression of hemichannels, where increased hemichannel activity/function reflects increased loading. The technique relies on the uptake of a membrane-impermeable fluorescent tracer through open hemichannels, and can be used to compare channel activity between cell monolayers cultured under different conditions, e.g. control versus disease. Other techniques, such as biotinylation and electrophysiology can measure cell surface expression and hemichannel open probability respectively, however, carboxyfluorescein uptake provides a simple, rapid and cost-effective method to determine hemichannel activity in vitro in multiple cell types.
Graphic abstract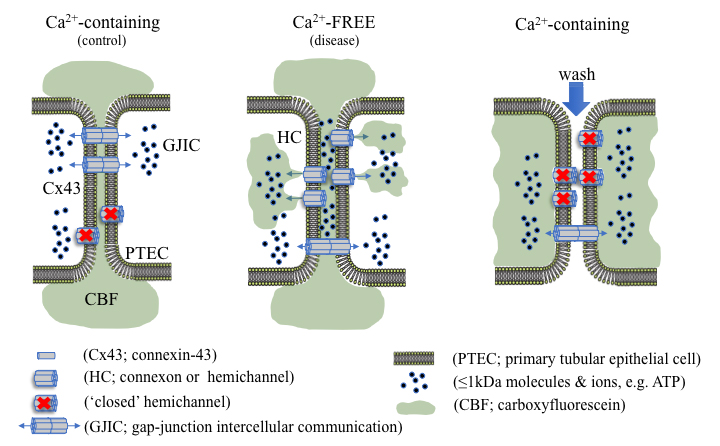
Using dye uptake to measure hemichannel activity
Background
Connexins (Cx) are integral transmembrane proteins that oligomerise into connexons at the cell surface. Connexons dock with similar hexameric protein complexes on adjacent cells to form a bidirectional conduit for gap junction intercellular communication (GJIC; Bosco et al., 2011). Gap junctions play an important role in synchronizing cellular activity and allow for the direct exchange of small ions, metabolites and secondary messengers, crucial for cellular function, survival and tissue homeostasis (Spray and Hanani, 2019). Under normal conditions, undocked connexons, known as hemichannels, remain mostly closed; however, in a pathophysiological context hemichannel-mediated intercellular communication can predominate, permitting the release of 1-2 kDa molecules, including adenosine triphosphate (ATP) into the extracellular milieu (Solini et al., 2015; Hills et al., 2018; Taruno, 2018; Siamantouras et al., 2019). Altered hemichannel activity has previously been linked to inflammation, fibrosis and the progression of various disease states (Roy et al., 2017; Hills et al., 2018; Cea et al., 2020; Sáez et al., 2020). Consequently, quantitative characterisation of hemichannel activity can inform on the onset and progression of disease, e.g., chronic kidney disease (CKD), along with highlighting potential targets for therapeutic intervention (Price et al., 2020).
Depending on the constituent connexin isoform, several factors are known to open hemichannels, including temperature, pH, enteric pathogenic infections and low extracellular calcium ([Ca2+]e) (Trexler et al., 1999; Ceelen et al., 2011; Lopez et al., 2016; Pinto et al., 2017). Whilst various methods can determine expression and localisation of connexin hemichannel subunits at the cell surface, e.g., biotinylation combined with immunoblot analysis and immunocytochemistry respectively, these do not provide a measure for functional changes in connexin-mediated cell communication. In recent years, a range of different methods have been used to evaluate the functional implications of altered hemichannel activity and/or function (Johnson et al., 2016). One of these techniques involves electrophysiological characterisation using whole-cell patch clamp recordings. Although dual whole-cell voltage recordings offer a highly attractive method to attain vast amounts of information on hemichannel permeability selectivity, opening/closing kinetics, open probability latency and presence of subconductance states, it is not without its drawbacks (Brokamp et al., 2012). Not only is this technique highly complex, the membrane potential needed to gate hemichannels is cell-type specific, a difficult hurdle to overcome (Veenstra, 2001). Quantification of hemichannel-permeable molecules into the local extracellular environment (e.g., ATP release) via luminescence based assays is much simpler, yet provides minimal, indirect information with regards to hemichannel activity and would benefit from the use of potentially costly general or local connexin inhibitors (Musil and Goodenough, 1991; Brokamp et al., 2012; Sáez and Green, 2018; Price et al., 2020). Although these techniques provide excellent methodological approaches for identifying cell-surface connexin expression and hemichannel open probability, it is the use of simpler hemichannel-permeable, membrane impermeant dye uptake studies that are routinely used (Johnson et al., 2016). Dye uptake studies make use of controlled parameters and utilise altered [Ca2+]e to gate hemichannels, providing a simple, efficient and reliable procedure. Several fluorescent tracers, including Lucifer Yellow, ethidium bromide, 4’,6-diamidino-2-phenylindole (DAPI), propidium iodide (PI), and 5(6)-carboxyfluorescein can be used to quantify hemichannel activity, with the extent of dye uptake proportional to the number of active cell-surface hemichannels (Lopez et al., 2016). Unfortunately, the majority of these tracers are hazardous and carcinogenic and require careful handling; however, this step-by-step protocol utilises the non-hazardous, 376 Da polyanionic fluorescent probe, 5(6)-carboxyfluorescein, to quantitively measure hemichannel activity in proximal tubular epithelial cells in vitro.
Materials and Reagents
50 ml Falcon tubes
Fluorodish, 35 mm (World Precision Instruments, catalog number: FD35-100 )
Pasteur pipette, 4 ml (Sigma-Aldrich, catalog number: BR747770 )
Clonal human kidney proximal tubule (HK-2) epithelial cell line (American Type Culture Collection, catalog number: CRL-2190 )
Dulbecco’s Modified Eagle Medium/Ham’s F-12 (Fisher Scientific, catalog number: 31331093 )
Dulbecco’s Modified Eagle Medium (Fisher Scientific, catalog number: 11966025 )
Ham’s F-12 (Fisher Scientific, catalog number: 31-765-035 )
Penicillin (100 IU/ml)-streptomycin (100 µg/ml) (Sigma-Aldrich, catalog number: P4458-100mL )
Epidermal growth factor (5 ng/ml) (Sigma-Aldrich, catalog number: E9644-.5mg )
Transforming Growth Factor-beta1 (TGF-β1), stored at -20 °C (Sigma-Aldrich, catalog number: T1654 )
Foetal calf serum (Fisher Scientific, catalog number: 10500064 )
Sodium chloride (NaCl) (Fisher scientific, catalog number: S/3160/65 )
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P3911 )
Magnesium sulphate heptahydrate (MgSO4·7H2O) (Sigma-Aldrich, catalog number: 230391 )
Sodium phosphate dibasic dihydrate (Na2HPO4·2H2O) (Sigma-Aldrich, catalog number: 71643 )
Potassium dihydrogen phosphate (KH2PO4) (Sigma-Aldrich, catalog number: 1.04873 )
Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S5761 )
HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (Sigma-Aldrich, catalog number: H3375 )
Calcium chloride dihydrate (CaCl2·2H2O) (Sigma-Aldrich, catalog number: 223506 )
D-glucose (C6H12O6) (Sigma-Aldrich, catalog number: G8270 )
Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: S8045 )
Hydrochloric acid (HCl) (Sigma-Aldrich, catalog number: 320331 )
EGTA (ethylene glycol-bis(β-aminoethyl ether)-N,N,N’,N’-tetraacetic acid) (Sigma-Aldrich, catalog number: E3889 )
Cell culture
Growth culture medium, stored at 4 °C (see Recipes)
Low (5 mM) glucose medium, stored at 4 °C (see Recipes)
Low (5 mM) glucose serum free medium, stored at 4 °C (see Recipes)
5(6)-Carboxyfluorescein, stored at RT (Sigma-Aldrich, catalog number: 21879 ) (see Recipes)
1× Calcium-containing balanced salt solution, stored at 4 °C (see Recipes)
1× Calcium-free balanced salt solution, stored at 4 °C (see Recipes)
Equipment
Small, unstirred, heated water bath (37 °C) (Grant instruments Ltd, catalog number: 600142003 )
Clifton 14 L, heated water bath (37 °C) (Nickel-electro Ltd, catalog number: NE1-14 )
Humidified incubator: 37 °C, pH 7.4, 5% CO2 (CO2 incubator, Panasonic, catalog number: MCO-170AICD )
Linus photonics metafluor imaging workbench (Technical Manufacturing Corporation, catalog number: 63-530 )
Photometrics CoolSNAP HQ CCD monochrome camera with power supply (Roper Scientific, catalog number: A01C881022 )
Zeiss Axiovert 200 research inverted fluorescence microscope with heated stage (Carl Zeiss Ltd, catalog number: B40-080 )
Zeiss XBO 75 microscope lamp housing with EBX 75 isolated power supply (Carl Zeiss Ltd, catalog number: B40-065 )
Lambda 10-2 optical filter changer (Sutter Instrument, catalog number: LB10-2 )
Tempcontrol 37 (Carl Zeiss Ltd, catalog number: 37-2 )
Dell precision PC (Dell, catalog number: T3500 )
Liyama prolite PC monitor (Iiyama, catalog number: E2472HD )
Sonomatic sonicator (Jencon scientific Ltd, catalog number: E0375 )
Software
Metamorph v7.7.5.0 software (available from https://www.moleculardevices.com/products/cellular-imaging-systems/acquisition-and-analysis-software/metamorph-microscopy#gref) (Meta imaging series by Molecular Devices Inc)
Fiji (ImageJ) v2.1.0 software (available from https://fiji.sc/) (ImageJ studio Ltd)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Potter, J. A., Price, G. W., Cliff, C. L., Williams, B. M., Hills, C. E. and Squires, P. E. (2021). Carboxyfluorescein Dye Uptake to Measure Connexin-mediated Hemichannel Activity in Cultured Cells. Bio-protocol 11(3): e3901. DOI: 10.21769/BioProtoc.3901.
分类
癌症生物学 > 通用技术 > 细胞生物学试验 > 细胞粘附
细胞生物学 > 基于细胞的分析方法 > 细胞间的相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link