- EN - English
- CN - 中文
Multiplex T-cell Stimulation Assay Utilizing a T-cell Activation Reporter-based Detection System
利用基于T细胞活化报告基因的检测系统进行多重T细胞刺激测定
(*contributed equally to this work) 发布: 2021年01月20日第11卷第2期 DOI: 10.21769/BioProtoc.3883 浏览次数: 7150
评审: Alessandro DidonnaShuiqing HuShalini Low-Nam

相关实验方案

基于 T 细胞的平台,用于对肿瘤浸润 T 细胞的单细胞 RNA 测序数据集中鉴定的 T 细胞受体进行功能筛选
Aaron Rodriguez Ehrenfried [...] Rienk Offringa
2024年04月20日 6415 阅读

研究免疫调控血管功能的新实验方法:小鼠主动脉与T淋巴细胞或巨噬细胞的共培养
Taylor C. Kress [...] Eric J. Belin de Chantemèle
2025年09月05日 3559 阅读
Abstract
Immune tolerance and response are both largely driven by the interactions between the major histocompatibility complex (MHC) expressed by antigen presenting cells (APCs), T-cell receptors (TCRs) on T-cells, and their cognate antigens. Disordered interactions cause the pathogenesis of autoimmune diseases such as type 1 diabetes. Therefore, the identification of antigenic epitopes of autoreactive T-cells leads to important advances in therapeutics and biomarkers. Next-generation sequencing methods allow for the rapid identification of thousands of TCR clonotypes from single T-cells, and thus there is a need to determine cognate antigens for identified TCRs. This protocol describes a reporter system of T-cell activation where the fluorescent reporter protein ZsGreen-1 is driven by nuclear factor of activated T-cells (NFAT) signaling and read by flow cytometry. Reporter T-cells also constitutively express additional pairs of fluorescent proteins as identifiers, allowing for multiplexing of up to eight different reporter T-cell lines simultaneously, each expressing a different TCR of interest and distinguishable by flow cytometry. Once TCR expression cell lines are made they can be used indefinitely for making new T-cell lines with just one transduction step. This multiplexing system permits screening numbers of TCR-antigen interactions that would otherwise be impractical, can be used in a variety of contexts (i.e., screening individual antigens or antigen pools), and can be applied to study any T-cell-MHC-antigen trimolecular interaction.
Keywords: Antigens (抗原)Background
The interactions between T-cells, antigen-presenting cells (APCs), and their cognate antigens are central events in autoimmune diseases such as type 1 diabetes (Michels et al., 2017; Mann et al., 2020), as well as other immune-based responses including tumor suppression. High-throughput sequencing of the α and β chain genes for T-cell receptors (TCRs) from single T-cells via next-generation sequencing (NGS) allows for the rapid identification of thousands of TCRs that are potentially involved in disease onset and progression. Determining the biologically relevant antigens recognized by a large number of TCRs is an important need towards understanding disease pathogenesis and ultimately developing antigen-specific immunotherapies and diagnostics for personalized medicine. Techniques that enable screening of immense numbers of potential antigens, such as combinatorial peptide libraries, require stable T-cell lines that can be expanded reliably without losing sensitivity or reproducibility. The system described here achieves this purpose by generating T-cell ‘avatars’ expressing human TCRs in a murine T-cell hybridoma line that can be easily grown while retaining response to cognate antigens.
Flow cytometric methods, in which a fluorescent reporter gene is driven by response-specific transcription factors such as nuclear factor of activated T-cells (NFAT), sensitively detect T-cell activation (Figure 1) and can distinguish each cell population by marking with fluorescent proteins (FPs), allowing for the simultaneous evaluation of multiple cell populations. This multiplexing approach reduces the labor and reagents needed for detection of response to antigen when large numbers of TCRs are to be analyzed (Mann et al., 2020). Here we describe an NFAT-driven fluorescent reporter system and T-cell multiplexing strategy for large-scale screening of TCR and peptide-MHC complex interactions, to surmount this limiting step in determination of TCR antigen specificity.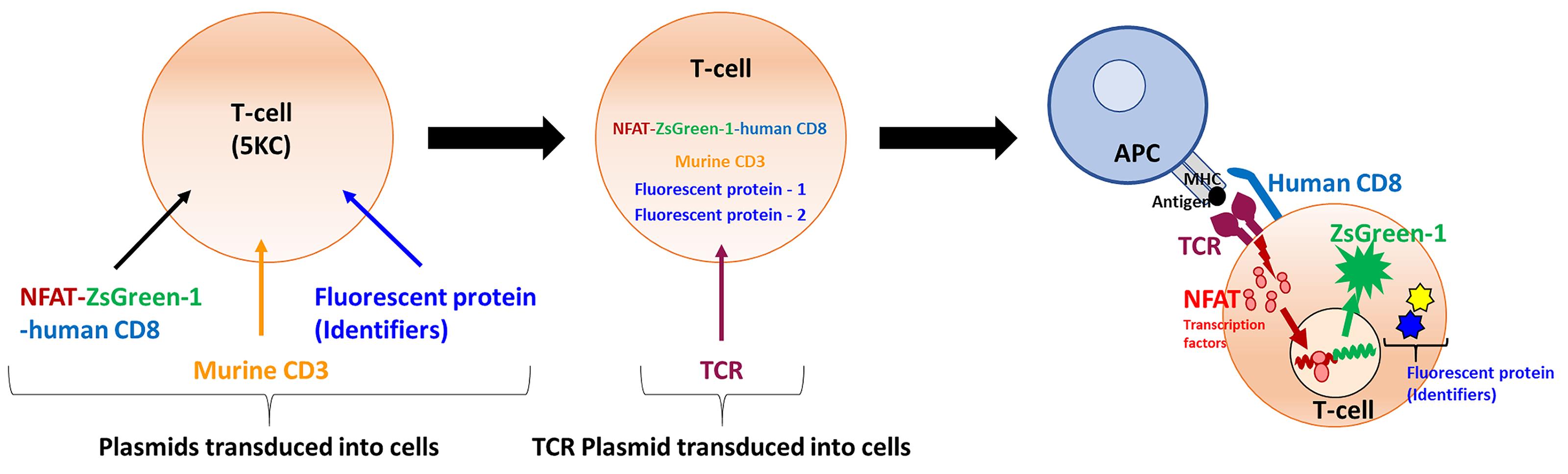
Figure 1. Principle of the assay – strategy of reporter T-cell generation. Four vectors for making TCR expression cell lines are transduced into 5KC α-β- cells (T-hybridoma cells lacking endogenous TCR expression). The first vector contains the NFAT-binding sequence followed by the fluorescent ZsGreen-1 gene, and the human CD8 co-receptor gene driven by the PGK promoter. The second vector encodes the Murine CD3. The third and fourth vectors contain fluorescent protein (FP) genes as identifiers. The TCR expression cell line is then transduced with a vector encoding TCR alpha and beta chain genes to generate functional TCR reporter cells. When the TCR reporter cell is activated upon the recognition of a peptide presented by an antigen presenting cells (APC), NFAT transcription factor proteins bind to the NFAT-binding site and promote the ZsGreen-1 gene expression, which is detected by flow cytometry. By using 8 combinations of FP identifiers, mixtures of 8 different TCRs can by assayed in the same reaction well.
In this procedure, murine T-hybridoma cells without endogenous TCR expression (5KC α-β-, White et al., 1993) are used to express the TCR α and β chains corresponding to a specific clonotype. 5KC α-β- cells are derived from murine CD4 T-cells and express the murine CD4 antigen. Therefore, human CD8 (hCD8) or human CD4 (hCD4) genes need to be introduced into 5KC α-β- for the screening of human TCRs. In this protocol, in which we describe experiments to screen hCD8 TCR antigen specificity, T-cell lines are created by introduction of the following components: (1) NFAT-driven ZsGreen-1 reporter vector that also contains hCD8 genes, (2) additional murine CD3 genes to enhance TCR expression, (3) fluorescent protein identifiers, allowing up to eight cell lines to be multiplexed in one reaction well and distinguished via flow cytometry, and (4) chimeric TCR α and β chain genes composed of the human TCR variable region of interest and the mouse constant region (Figure 1). Of note, once cell lines that are introduced with the first three components without TCR genes are generated, such parental cell lines can be used indefinitely to express different TCRs. By assaying eight T-cell lines together, requirements of time, labor and reagents for antigen screening (media, peptides) are substantially reduced, permitting the analysis of large numbers of TCRs for the response to thousands of antigens. This approach can be applied to human or murine T-cells, as well as those with different major histocompatibility complex (MHC) restrictions, by replacing hCD8 with other molecules, such as hCD4.
Materials and Reagents
5-ml serological pipettes (Fisherbrand, catalog number: 13-678-11D )
10-ml serological pipettes (Fisherbrand, catalog number: 13-678-11E )
25-ml serological pipettes (Fisherbrand, catalog number: 13-678-11 )
Filtered 10 μl micropipette tips (MultiMax, catalog number: JM2015-108 )
Filtered 200 μl micropipette tips (MultiMax, catalog number: B-2104-SH )
Filtered 1,250 μl micropipette tips (MultiMax, catalog number: B-2105-L )
15-ml conical centrifuge tubes (TrueLine, catalog number: TR2000 )
50-ml conical centrifuge tubes (TrueLine, catalog number: TR2003 )
1.5-ml Optimum Microcentrifuge tubes (Life Science Products, catalog number: 8215-0500N )
96-well PCR plates (Fisherbrand, catalog number: 14230232 )
Sterile 2 mil Seal Film Tape for PCR (Life Science Products, catalog number: ST-3099 )
Foil PCR plate seals (Fisher Scientific, Axygen, catalog number: 14-222-343 )
96-well cell culture plates (Falcon, catalog number: 353077 )
12-well cell culture plates (Falcon, catalog number: 353043 )
6-well cell culture plates (Falcon, catalog number: 353046 )
10-cm tissue culture dishes (Falcon, catalog number: 353003 )
T25 tissue culture flasks (Thermo Scientific Nunc, catalog number: 156367 )
T75 tissue culture flasks (Thermo Scientific Nunc, catalog number: 156499 )
5-ml disposable syringes (BD, catalog number: 1482945 )
Millex HV syringe filter, 0.45 μm (EMD Millipore, catalog number: SLHV033RS )
Syringe filters, 0.2 μm (Corning, catalog number: 431219 )
Wide-bore filter tips (ART, catalog number: 212362C )
1.8-ml cryotubes (Thermo Fisher Nunc, catalog number: 377267 )
Sterile 14-ml round-bottom tubes (Falcon, catalog number: 352059 )
Caplugs sterile polypropylene culture tubes, 4 ml (Evergreen Scientific, catalog number: 05-551-2 )
Rapid-Flow Sterile Disposable Filter Units, 500 ml, 0.2 μm (Nalgene, catalog number: 569-0020 )
Blunt cannulas, 15-gauge (Covidien Monoject, catalog number: 22-652-085 )
100 μm cell strainer (Falcon, catalog number: 352360 )
10 cm Petri dishes (Fisherbrand, catalog number: 08-757-12 )
Synthetic double-stranded DNA fragments (TWIST Bioscience or Integrated DNA Technologies, custom)
Cell lines
Vectors
8xNFAT-ZsG-hCD8 (Addgene, catalog number: 153417 )
Murine CD3 WTdelta-F2A-gamma-T2A-epsilon-P2A-zeta pMIA II [CD3-AM] (Addgene catalog number: 52093 )
CD3-2A_pMI-LO [CD3-LO] (Addgene, catalog number: 153418 )
pMSCVII (Addgene, catalog number: 162750 )
pMSCVII-AM [pMSCV-AM] (Addgene, catalog number: 162751 )
pMSCVII-LO [pMSCV-LO] (Addgene, catalog number: 162754 )
pMSCVII-BFP [pMSCV-BFP] (Addgene, catalog number: 162755 )
pMSCVII-tdTomato [pMSCV-TM] (Addgene, catalog number: 162752 )
pMSCVII-E2Crimson [pMSCV-CR] (Addgene, catalog number: 162756 )
pMSCVII-mChe [pMSCV-mChe] (Addgene, catalog number: 162753 )
MBC1-null (Addgene, catalog number: 162748 )
MBC2-null (Addgene, catalog number: 162749 )
CaP2AIII_pK18 [CaP2AIII] (Addgene, catalog number: 153421 )
pUS (Addgene, catalog number: 153416 )
A2_uSFFV (Addgene, catalog number: 153415 )
pCL-ECO (Addgene, catalog number: 12371 )
pMD2.G (Addgene, catalog number: 12259 )
psPAX2 (Addgene, catalog number: 12260 )
Restriction enzymes (New England Biolabs)
BspEI (NEB, catalog number: R0540S )
HindIII (NEB, catalog number: R0104S )
MfeI (NEB, catalog number: R0589S )
HpaI (NEB, catalog number: R0105S )
EcoRI (NEB, catalog number: R0101S )
XhoI (NEB, catalog number: R0146S )
MscI (NEB, catalog number: R0534S )
AleI-v2 (NEB, catalog number: R0685S )
SalI (NEB, catalog number: R0138S )
BglII (NEB, catalog number: R0144S )
MluI-HF (NEB, catalog number: R3198S )
SbfI (NEB, catalog number: R0642S )
Primers (Integrated DNA Technologies)
BspEI-F (GCGGGCGCCCGAAGGTCCT)
Hind Seq-R (GGCTTCAGCTGGTGAAGCTT)
CD3-Seq (ATGGCCTTTACCAGGGTCTC)
SalI-Seq (GCATGCTAGCTATAGTTCTAGAG)
5pMIG (GCCTCCTCTTCCTCCATCCG)
3LTR-2 (AATTTGCGCATGCTAGCTATAG)
TRAC1-R (CAGGCAGAGGGTGCTGTCCTG)
5MAC3 (AGAGACCAACGCCACCTACC)
3TRBC (CAAGGAGACCTTGGGTGGAG)
Cas9-S14 (CACATAGCGTAAAAGGAGC)
Cas9-S16 (AAGAGCTCACAACCCCTCAC)
P2A-R (GAAGTTCGTGGCTCCGGAG)
PGK-F2 (CATTCCACATCCACCGGTAG)
P2A-F (CTCCGGAGCCACGAACTTC)
P2A-R2 (GGGACCGGGGTTTTCTTCCAC)
3LTR (GCAAAATGGCGTTACTTAAGC)
IRES-Seq (CTGATCTGGGGCCTCGGTGC)
LB broth (Gibco, catalog number: 10855-021 )
LB agar (BD Difco, catalog number: 244520 )
Ampicillin Ready Made Solution (Sigma-Aldrich, catalog number: A5354-10ML )
QIAQuick Gel Extraction Kit (Qiagen, catalog number: 28704 )
QIAQuick PCR Clean-up Kit (Qiagen, catalog number: 28104 )
Qiagen Minelute Gel Extraction Kit (Qiagen, catalog number: 28604 )
NEBuilder HiFi DNA Assembly Master Mix (NEB, catalog number: E2621L )
NEB 5-alpha Competent E. coli (HE) (NEB, catalog number: C2987H )
Agarose (Fisherbrand, catalog number: BP160-500 )
Ethidium bromide, 10 mg/ml (Fisher Scientific, Invitrogen, catalog number: 15-585-011 )
PreMix D (Lucigen, catalog number: MO7205D )
Taq DNA Polymerase (Sigma, catalog number: D1806-20X250UN )
100 bp DNA ladder (Fisher Scientific, Invitrogen, catalog number: 15628-019 )
1 Kb Plus DNA ladder (Fisher Scientific, Invitrogen, catalog number: 10-787-018 )
Qiagen Plasmid Plus Midi Kit (Qiagen, catalog number: 12943 )
Fetal bovine serum (FBS) (Atlanta Biologicals, catalog number: S11150H )
Lipofectamine 2000 (Invitrogen, catalog number: 11668-500 )
Molecular biology grade water (HyClone, catalog number: 3053801 )
Poly-D-lysine (PDL) hydrobromide lyophilized powder (Sigma-Aldrich, catalog number: P6407-10x5MG )
Sterile cell culture grade water (HyClone Water, Cell Culture Grade (Endotoxin-Free), catalog number: SH3052901 )
Phosphate-buffered saline (PBS), pH 7.4 (Gibco, catalog number: 10010023 )
Penicillin/streptomycin (pen/strep), sterile filtered (Sigma-Aldrich, catalog number: P0781 )
Trypsin-EDTA, 0.25% (Gibco, catalog number: 25200-056 )
Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, catalog number: 11965-092 )
Iscove’s Modified Dulbecco’s Medium (IMDM) (Gibco, catalog number: 12440079 )
RPMI 1640 medium, no phenol red (Gibco, catalog number: 11835030 )
MEM Non-essential Amino Acids Solution (MEM-NEAA) (100x) (Gibco, catalog number: 11140-050 )
HEPES Buffer, 1 M (Gibco, catalog number: 15630-080 )
Sodium Pyruvate, 100 mM (Gibco, catalog number: 11360-070 )
2-mercaptoethanol [14.3 M] (Sigma-Aldrich, catalog number: M3148 )
Dimethyl sulfoxide (DMSO) (Fisher Bioreagents, catalog number: BP231-100 )
Protamine sulfate powder (MP Biomedicals, catalog number: ICN19472901 )
PE-labeled anti-human CD8 antibody (Biolegend, clone SK1, catalog number: 344706 )
Anti-mouse CD3e antibody, functional grade (BD, clone 145-2C11, catalog number: 553057 )
PE-labeled anti-mouse CD3e antibody (BD, clone 145-2C11, catalog number: 553064 )
PE-labeled anti-HLA-ABC antibody (BD, clone G46-2.6, catalog number: 560964 )
Mouse CD3e microbead kit (Miltenyi Biotec, catalog number: 130-094-973 )
Zombie NIR Fixable Viability Kit (BioLegend, catalog number 423105 )
Transfection medium (see Recipes)
5KC medium (see Recipes)
Phoenix medium (see Recipes)
5KC Freezing Medium (see Recipes)
Phenol red-free RPMI (see Recipes)
Protamine sulfate, 5 mg/ml solution (see Recipes)
PDL, 0.1 mg/ml (see Recipes)
LB-ampicillin Broth (see Recipes)
LB-ampicillin plates (see Recipes)
Equipment
Pipet controller (Fisherbrand, catalog number: FB14955202 )
Micropipettes (Fisherbrand Elite Kit, catalog number: 14-388-100 )
Multichannel pipettors (Fisherbrand Elite series)
Water bath (Fisher Scientific, Corning, 6-L digital waterbath, catalog number: 07-202-156 )
Ultra-low freezer (Thermo Scientific, TSX series, catalog number: TSX50086A )
Dry bath (Fisherbrand, Isotemp digital heat double-stranded DNA fragment, catalog number: 88-860-021 )
Electrophoresis gel boxes (Fisher Scientific, Owl D4 Horizontal Electrophoresis System, catalog number: 09-528-205 )
Electrophoresis power supply (Fisherbrand, catalog number: FB200Q )
PCR Thermal Cycler (Thermo Fisher Scientific, Applied Biosystems ProFlex 96-well PCR System, catalog number: 4484075 )
NanoDrop 2000 spectrophotometer (Thermo Scientific, model: NanoDropTM 2000 )
Shaking incubator (New Brunswick Scientific, model: Innova 43 )
GelDoc Go Gel Imaging System (Bio-Rad, catalog number: 12009077 )
Microcentrifuge (Fisherbrand accuSpinTM Micro 17, catalog number: 13-100-675 )
Countess II automated cell counter (Invitrogen, catalog number: AMQAX1000 )
Countess II slides (Invitrogen, catalog number: C10228 )
Inverted light microscope (Olympus, model: IX53 )
Bench centrifuge (Thermo Scientific, SorvallTM LegendTM XT/XF Centrifuge and Rotor Packages, catalog number: 75-333-839 )
Biosafety BSL2 cabinet (NuAire, model: NU-425-400 )
CO2 Incubator (Fisherbrand Isotemp CO2 Incubators, catalog number: 11-676-600 )
Cytoflex (Beckman Coulter, https://www.beckman.com/flow-cytometry/instruments/cytoflex)
Mr. Frosty freezing container (Thermo Scientific, catalog number: 15-350-50 )
MS Columns (Miltenyi Biotec, catalog number: 130-042-201 )
OctoMACS Separator (Miltenyi Biotec, catalog number: 130-042-109 )
MilliQ water machine (Millipore-Sigma, lot number: F8BA18025 )
Software
Sequencher, Gene Codes Corp (http://www.genecodes.com/)
SnapGene version 5.0.4 (https://www.snapgene.com/)
FlowJo v10 (https://www.flowjo.com/)
Prism GraphPad (https://www.graphpad.com/)
NCBI nucleotide website (https://www.ncbi.nlm.nih.gov/nucleotide/)
FP database (https://www.fpbase.org/)
IMGT website (http://www.imgt.org/IMGTrepertoire/Proteins/index.php#B)
HLA database (https://www.ebi.ac.uk/ipd/imgt/MHC/allele.html)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Landry, L. G., Mann, S. E., Anderson, A. M. and Nakayama, M. (2021). Multiplex T-cell Stimulation Assay Utilizing a T-cell Activation Reporter-based Detection System. Bio-protocol 11(2): e3883. DOI: 10.21769/BioProtoc.3883.
分类
免疫学 > 免疫细胞功能 > 淋巴细胞
细胞生物学 > 基于细胞的分析方法 > 蛋白互作
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










