- EN - English
- CN - 中文
A Simple Microplate Assay for Reactive Oxygen Species Generation and Rapid Cellular Protein Normalization
用于活性氧生成和快速细胞蛋白质标准化的简单微孔板检测
发布: 2021年01月05日第11卷第1期 DOI: 10.21769/BioProtoc.3877 浏览次数: 9332
评审: Kuo-Ching "KC" MeiAnonymous reviewer(s)

相关实验方案
外周血中细胞外囊泡的分离与分析方法:红细胞、内皮细胞及血小板来源的细胞外囊泡
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
2025年11月05日 1435 阅读
Abstract
2',7'-dichlorofluorescein (DCF) and derivatives are commonly used as fluorescent indicators of a broad spectrum of reactive oxygen species (ROS) generation in cell-based assays. However, there are numerous challenges inherent to the utilization of DCF probes for intracellular microscopic analysis, including bleaching and probe efflux. Plate spectroscopy is comparatively simple and scalable compared to microscopy or flow cytometry-based acquisition, however is often subject to artefacts, including those introduced by thermal gradients and normalization methods. In this protocol we demonstrate a simple and sensitive plate spectrometry-based protocol utilizing the probes H2DCFDA and sulforhodamine B. The truncated sulforhodamine B assay (SRB) for cellular protein allows for a stable endpoint measurement of total cell population while also preserving morphology, can be combined or run in parallel with any other assay for normalization of readout to cell mass, and complemented by microscopic scoring of cell number and nuclear count. The oxidative stress and normalisation methods may enhance fields of research investigating cell differentiation, stress, or toxicity.
Graphical abstract
Background
Microplate assays offer a significant advantage over microscopy-based assays with regard to throughput, cost of analysis equipment, optimization and analysis time. The detection of reactive oxygen intermediates (ROS) generated by drugs in activated inflammatory cell types can be facilitated by fluorescent probes such as 2',7'-dichlorodihydrofluorescein diacetate (H2DCFDA) and dihydroethidium (DHE) in microscopy, plate spectroscopy and flow cytometry formats. Following deacetylation by intracellular esterases, H2DCF is oxidized by hydroxyl, carbonate, nitrogen or thiyl radicals to the green fluorescent DCF (Figure 1). As such DCF derivative probes are not only sensitive indicators of intracellular and drug-induced ROS generation but also prone to artefactual signal, including self-oxidation from H2O2 generated during oxidation, and photo-oxidation by an excitation source (Wojtala et al., 2014; Paige Davis Volk and Moreland, 2014). Although DCF probes do not result in formation of nucleic acid binding species in comparison to DHE, DCF is not retained intracellularly for extended periods of time, limiting its window of detecting ROS for microscopy or flow cytometry. We have found that ROS generation can be more sensitively detected by a comparatively simple experimental analysis mode of plate spectroscopy.
Figure 1. Mechanism of H2DCFDA ROS probe. Cell permeable H2DCFDA is cleaved by intracellular esterases to non-fluorescent H2DCF, which is oxidized by various ROS to green fluorescent DCF which can be detected by fluorescence plate spectroscopy.
Potentially overlooked artefactual distortions to signal in microplate format include uneven seeding, thermal gradient effects formed during heating, evaporation and background signal from small molecules. In this protocol, even seeding is addressed by a pre-incubation at ambient temperature (Lundholt et al., 2003). Fluorescent background signal contributed by small molecules or other reagents can be deducted with cell-free controls. While microplate edge wells are often replaced with water to reduce loss of volume even in humidified incubators, enclosure of microplates in a simple humidifying chamber can overcome edge well evaporation (Walzl et al., 2012). Thermal edge effects due to uneven heating can be addressed by use of metal heating blocks that align with base of microtitre plates and pre-heated reaction solutions (Shellman et al., 2004), or room temperature incubation where possible.
Cell-based plate spectroscopy assays such as the DCF assay typically require normalization to cell population per well. Microplate assay signal to population normalizations are often performed with the bicinchronic acid (BCA) assay. However the BCA assay is compromised by low concentrations of H2O2, and practical scalability is challenged due to the lack of endpoint, or stop reaction method (Baker, 1991). Nuclear DNA stains are often employed for normalization of cell populations when scored microscopically, but overestimate populations in plate spectroscopy format where there is high DNA condensation with cell death, particularly relevant to small molecule or radiation stress experiments. The sulforhodamine B (SRB) assay provides a stable endpoint measurement of the cell population by quantifying fixed cellular protein whilst preserving morphology and avoiding drug interactions (Skehan et al., 1990). SRB is an anionic aminoxanthene protein dye that under acidic conditions binds basic amino acid residues (Han et al., 2012). Numerous SRB assays have been presented involving typically hour long cold acidic protein precipitation by trichloroacetic acid (TCA), SRB staining and solubilization with numerous wash steps. However the fixation reaction by cold acid precipitation (Hayashi et al., 1999) and SRB staining has been reported to reach completion within minutes (Skehan et al., 1990). In this protocol the DCF assay is normalized to cellular protein by a TCA-SRB assay that combines fixation and staining steps (Figure 2).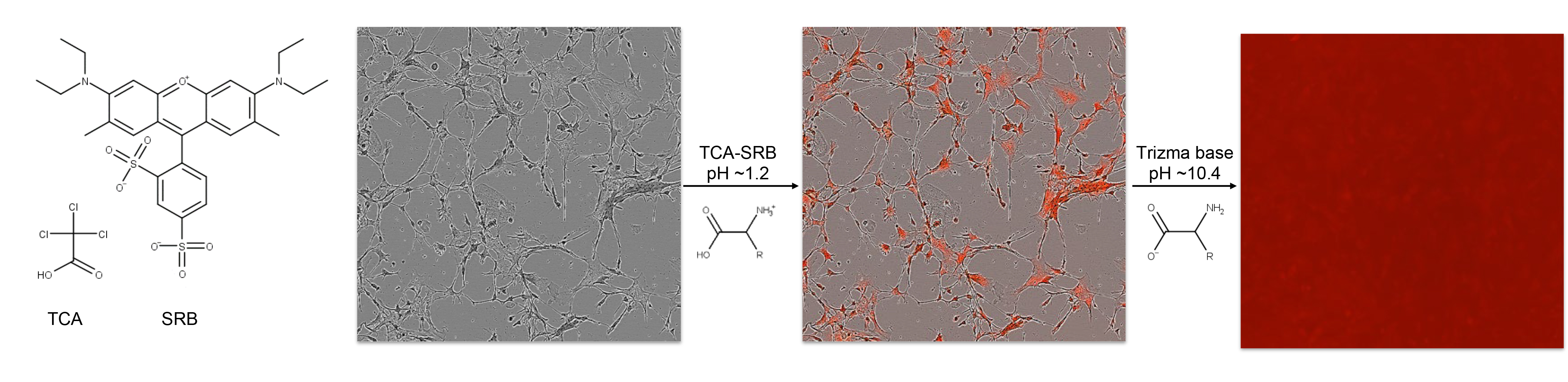
Figure 2. Rapid TCA-SRB assay schematic. Cell cultures are simultaneously fixed and stained by cold TCA-SRB. In acidic conditions SRB binds basic amino acid residues. After a wash step, SRB is solubilized in basic conditions and released into well contents, reflecting content of cellular protein per well.
The protocol is applicable to any adherent cell culture in microplate well format, and can be executed by any basic cell culture laboratory equipped with a fluorescent spectroscopy plate reader with green and red fluorescent channels (excitation 494/emission 522 and excitation 565/emission 586) (Figure 3). The rapid SRB assay can be used to normalize any other non-destructive cell-based assay readout. All reagents required are easily commercially available at very low cost per assay plate.
Figure 3. Protocol schematic. Cells are loaded with DCF-based probe, incubated for desired treatment period (e.g., 1 h or 24 h) before plate spectroscopy readout of DCF as an indication of ROS generation. To normalize DCF signal to cell population per well, a simultaneous acidic protein precipitation stage and cellular protein dye stage is performed before solubilization and spectroscopic readout of SRB. The TCA-SRB assay can be utilized to obtain relative cell populations for growth inhibition, cytotoxicity or to normalize other cell-based assay readouts.
Materials and Reagents
15 or 50 ml centrifuge tubes
96-well microplates (ideally black-walled, clear bottom)
DMEM/F-12, no phenol red (Thermo Fisher Scientific, Gibco, catalog number: 21041025 ), store at 2-8 °C
PBS Tablets (Thermo Fisher Scientific, Gibco, catalog number: 18912014 ), store at room temperature (RT)
H2DCFDA (Thermo Fisher Scientific, Life Technologies, catalog number: D399 ), store powder at -20 °C
Sulforhodamine B sodium salt (SRB) (Sigma-Aldrich, catalog number: S1402 ), store powder at RT
Trizma base (Sigma-Aldrich, catalog number: T1503 ), store powder at RT
Trichloroacetic acid (TCA) (Sigma-Aldrich, catalog number: T4885-1KG ), store powder at RT
Acetic acid (Sigma-Aldrich, catalog number: A6283 ), store liquid at RT
Extracellular matrix solution (e.g., thermally cross-linked ECM with cold 10 µg/ml Collagen I for fibroblasts, or 1% Engelbreth-Holm-Swarm (EHS) sarcoma from Matrigel or Geltrex for iPSC, neural stem cells, incubated at 37 °C for 30 min)
30% wt. Hydrogen peroxide
100% DMSO
MilliQ grade water
10 mM H2DCFDA in DMSO (see Recipes)
10% w/v TCA in water (see Recipes)
0.004% w/v SRB in 10% w/v TCA (TCA-SRB) (see Recipes)
10 mM Trizma in water (see Recipes)
Equipment
Assumes availability of typical cell culture equipment (CO2 incubator, centrifuge, biosafety cabinet, water bath, fridge and/or cold room, etc.)
BMG LABTECH POLARstar Omega plate reader or fluorescence plate spectrometer with similar fluorescence capacity in fluorescein channel and 494/522 nm and 565/586 nm
Multichannel pipette (~10 µl and ~100 µl range) and sterile reagent reservoirs
Plastic humidifier box
Corrosives waste container
Software
Microsoft Excel or other spreadsheet processing software
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Ng, N. and Ooi, L. (2021). A Simple Microplate Assay for Reactive Oxygen Species Generation and Rapid Cellular Protein Normalization . Bio-protocol 11(1): e3877. DOI: 10.21769/BioProtoc.3877.
分类
细胞生物学 > 基于细胞的分析方法 > 流式细胞术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link