- EN - English
- CN - 中文
Assessment of Diadenylate Cyclase and c-di-AMP-phosphodiesterase Activities Using Thin-layer and Ion Exchange Chromatography
使用薄层和离子交换色谱评估二腺苷酸环化酶和c-di-AMP-磷酸二酯酶的活性
(*contributed equally to this work) 发布: 2021年01月05日第11卷第1期 DOI: 10.21769/BioProtoc.3870 浏览次数: 4246
评审: Andrea PuharCristina Colomer-WinterAnonymous reviewer(s)
Abstract
All living cells use cyclic nucleotides as second messengers for signal sensing and transduction. Cyclic di-3′,5′-adenosine monophosphate (c-di-AMP) is primarily involved in the control of bacterial and euryarcheal osmoadaptation and is produced by diadenylate cyclases from two molecules of ATP. Specific phosphodiesterases hydrolyze c-di-AMP to the linear phosphoadenylate adenosine 5′-pApA or to AMP. Different methods including high-performance liquid chromatography (HPLC), thin-layer chromatography (TLC) and ion exchange chromatography (IEX) can be used to determine activities of c-di-AMP-synthesizing and degrading enzymes. Here, we describe in detail the TLC and IEX methods adapted for characterization of the diadenylate cyclase DisA and the phosphodiesterase AtaC from Streptomyces venezuelae. TLC allows quick and easy separation of radioactive-labeled substrates and products, while IEX avoids utilization of potentially hazardous radioactive substrates and can be used as a good substitute if an HPLC system is not available. Unlike in TLC assays, samples cannot be analyzed in parallel by using the IEX assay, thus it is more time consuming.
Keywords: c-di-AMP (环二腺苷酸)Background
Cyclic nucleotide second messengers are key molecules in prokaryotic and eukaryotic signaling pathways. Cyclic di-3′,5′-adenosine monophosphate (c-di-AMP) is a bacterial second messenger with many important functions, such as regulation of osmolyte homeostasis, cell wall metabolism, biofilm formation, DNA integrity, sporulation, virulence and growth (Fahmi et al., 2017). It is of particular interest as it is present in many human pathogens, such as Staphylococcus aureus, Mycobacterium tuberculosis and Streptococcus pneumoniae and was found to be essential for most species under normal growth conditions (Woodward et al., 2010; Corrigan et al., 2011; Luo and Helmann, 2012; Mehne et al., 2013; Whiteley et al., 2015). Intriguingly, also accumulation of c-di-AMP leads to growth defects (Bai et al., 2013; Mehne et al., 2013; Latoscha et al., 2020). Thus, the levels of this second messenger have to be tightly regulated. c-di-AMP is synthesized from two molecules of ATP by diadenylate cyclases (DAC) and degraded by specific phosphodiesterases (PDE) to 5′-pApA and/or two AMP molecules (Figure 1) (Fahmi et al., 2017; Yin et al., 2020).
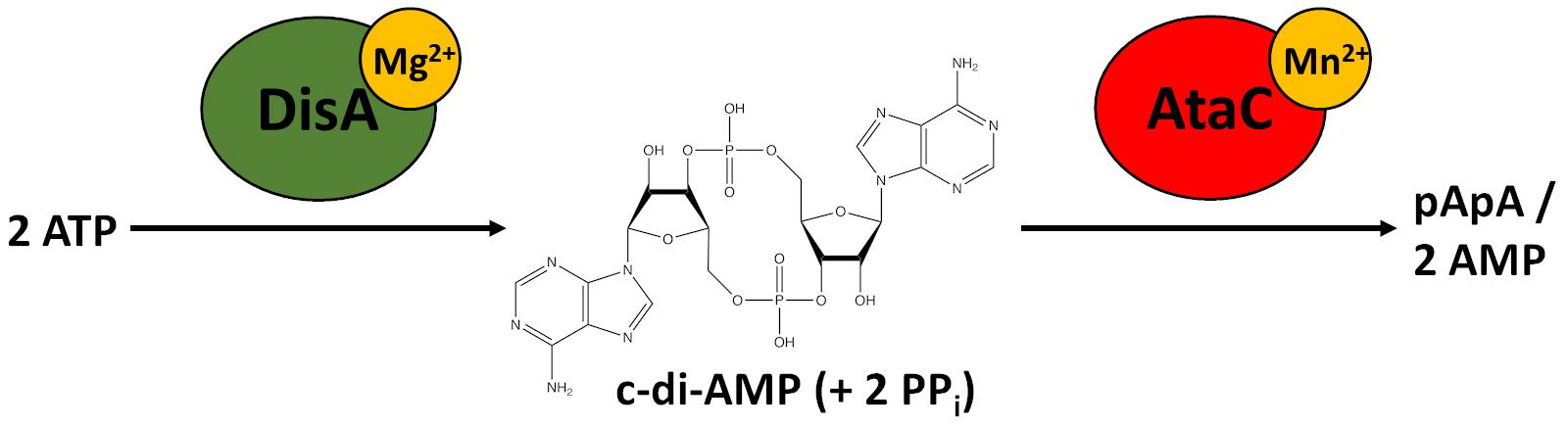
Figure 1. Synthesis and degradation of c-di-AMP in S. venezuelae. The diadenylate cyclase DisA uses magnesium ions (Mg2+) as cofactors to synthesize c-di-AMP from two ATP molecules releasing two pyrophosphates (PPi) as a byproduct (Witte et al., 2008). c-di-AMP is degraded to 5′-pApA and two AMP molecules by the phosphodiesterase AtaC, which requires manganese ions (Mn2+) as cofactors (Latoscha et al., 2020) .
Synthesis of c-di-AMP by DAC domains was identified in five different protein types, termed DisA, CdaA, CdaS, CdaM and CdaZ. Most bacteria contain either DisA or CdaA for c-di-AMP synthesis, while some species, exclusively belonging to the genus Bacillus possess DisA, CdaA and CdaS in parallel (Commichau et al., 2019). Enzymatic activity of these proteins can be affected by different conditions. The most widespread DAC, CdaA, is regulated by the extracytoplasmic regulator CdaR and the phosphoglucosamine mutase GlmM (Tosi et al., 2019; Gibhardt et al., 2020). In contrast, DisA synthesizes c-di-AMP constitutively unless it binds branched or damaged DNA (Witte et al., 2008), which results in a sporulation delay (Bejerano-Sagie et al., 2006; Oppenheimer-Shaanan et al., 2011). Thus, the synthesis of c-di-AMP can be affected by many different environmental conditions, which are still not understood in detail. For c-di-AMP degradation, two major classes of specific PDEs were identified so far, referred to as DHH-type and HD-type PDEs, which are termed according to the conserved catalytic amino acid motifs Asp-His-His and His-Asp in their respective active sites. DHH-type PDEs are further divided into multidomain membrane-coupled proteins like GdpP (Pde1) and soluble standalone catalytic domain proteins of the DhhP-type (Pde2) (Rao et al., 2010; Bai et al., 2013; Ye et al., 2014). The HD-type PDE is a membrane-coupled member of the 7TMR-HD family (7 transmembrane domain receptor with HD domain), termed PgpH (Gundlach et al., 2015; Huynh et al., 2015). Both GdpP and PgpH exclusively hydrolyze c-di-AMP into 5′-pApA, which is further degraded into AMP by DhhP or nano-RNases, such as NrnA. DhhP PDEs often occur in parallel to GdpP or PgpH in one species and were shown to be responsible for the second degradation step from 5′-pApA to AMP (Bowman et al., 2016; Drexler et al., 2017; Konno et al., 2018). In contrast, some species, such as M. tuberculosis and Mycobacterium smegmatis only contain DhhP homologs, which were shown to hydrolyze both c-di-AMP and 5′-pApA (Tang et al., 2015; He et al., 2016). However, many species from the phylum Actinobacteria, including streptomycetes, do not possess any of the major c-di-AMP-specific PDE classes (Corrigan and Gründling, 2013; Yin et al., 2020). Instead, Streptomyces venezuelae utilizes an alternative PDE for c-di-AMP degradation. In our recent study, we applied different biochemical approaches to characterize DisA, the sole DAC domain protein encoded in Actinobacteria, as well as AtaC (Actinobacterial PDE targeting c-di-AMP), which is the founding member of a new family of c-di-AMP-specific PDEs and is mainly present in Actinobacteria (Latoscha et al., 2020).
For an accurate characterization of proteins in this signaling pathway, robust and sensitive assays for substrate specificity are required. In the present protocol, we describe two optimized methods for the analysis of c-di-AMP synthesis or degradation: the thin-layer chromatography assay (TLC) of enzymatic assays with radiolabeled nucleotides and the ion exchange chromatography assay (IEX).
The TLC assay was established initially to study the activities of Caulobacter crescentus diguanylate cyclase (DGC) PleD and c-di-GMP-specific PDE CC3396 (Paul et al., 2004; Christen et al., 2005) based on a method published by Ross et al. (1987). Detailed protocols on DGC and c-di-GMP-specific PDE radioactive assays coupled with TLC were published recently (Kazmierczak, 2017a and 2017b). In the last years, enzymatic assays with radiolabeled nucleotides coupled with TLC have been successfully used for characterization of c-di-GMP-specific enzymes from Gram-negative and Gram-positive bacteria (Kazmierczak et al., 2006; Bordeleau et al., 2011; Lindenberg et al., 2013; Al-Bassam et al., 2018) as well as of the DAC DisA from Bacillus subtilis and Thermotoga maritima (Witte et al., 2008; Torres et al., 2019). In contrast to other methods like high-performance liquid chromatography (HPLC), the TLC assay allows separation of substrate and products from multiple reactions at the same time. Moreover, a direct conclusion about substrate specificity can be drawn if the reaction was supplemented with cold nucleotides as competitors for enzymatic activity (Paul et al., 2004; Christen et al., 2005; Lindenberg et al., 2013). If required, a quantification of radioactive product formation can be performed since only radiolabeled nucleotides, but not the unlabeled competitors, are visualized after development of the TLC (Ross et al., 1987; Paul et al., 2004; Christen et al., 2005). In our recent study, we used the TLC assay to analyze both synthesis of c-di-AMP by S. venezuelae DisA and specificity of c-di-AMP degradation by AtaC (Latoscha et al., 2020).
IEX was used as an independent method to further characterize the activity of AtaC (Latoscha et al., 2020). In this assay, negatively charged molecules (e.g., nucleotides) bind to a positively charged column material and can be eluted by a salt gradient. Depending on their binding affinity to the column, the molecules elute at different salt concentrations and can be separated. The assay is of particular interest if other chromatography systems for small molecules, such as reverse-phase HPLC (RP-HPLC) are not available. Cyclic dinucleotides and their related reaction intermediates 5’-pNpN or NMP were first separated by ion exchange chromatography by Li et al. (2013) to analyze the reaction products of cyclic GMP-AMP synthase (cGAS). The method was further used by Luecke et al. (2017) and the respective assay was described in detail by Holleufer and Hartmann (2018). The method was adapted for the characterization of a PDE in the degradation pathway of c-di-AMP (Drexler et al., 2017) and was slightly amended for the present assay for the characterization of AtaC (Latoscha et al., 2020). In principle, this IEX assay can be used to analyze the activity of most cyclic dinucleotide synthesizing or degrading enzymes. However, a RP-HPLC system provides a faster and more precise separation of the samples and if it is available, then RP-HPLC would be the preferred chromatographic method to use, as described in several reports (Oppenheimer-Shaanan et al., 2011; Bai et al., 2012; Witte et al., 2013; Huynh et al., 2015; Tang et al., 2015).
Materials and Reagents
Material for buffer exchange (see Notes)
Locking clips, 45 mm (Carl Roth, catalog number: H277.1 )
Dialysis membrane Spectra/Por 7 MWCO 10,000, 45 mm (Carl Roth, catalog number: E872.1 )
Glass pipettes: 5, 10, 20 and 25 ml (Labdirect, catalog numbers: 021.01.005 , 355.050.110 , 355.050.120 , 355.050.125 )
Pipette tips (Sarstedt, catalog numbers: 70.760.012, 70.762, 70.3020 and Biozym, catalog number: VZ0001X )
1.5 ml reaction tubes (Sarstedt, catalog number: 72.690.001 )
Double-distilled water (ddH2O) (Kerndl, catalog number: 22501 )
Magnesium chloride hexahydrate (MgCl2) (Carl Roth, catalog number: 2189.1 )
Manganese (II) chloride tetrahydrate (MnCl2) (Carl Roth, catalog number: T881.1 )
Sodium chloride (NaCl) (VWR, catalog number: 27810.295DB )
Tris-base (Carl Roth, catalog number: 4855.2 )
Glycerol (Carl Roth, catalog number: 3783.2 )
β-mercaptoethanol (Sigma-Aldrich, catalog number: M6250 )
Material for protein concentration determination (see Notes)
1.5 ml reaction tubes (Sarstedt, catalog number: 72.690.001 )
Spacer plates and short plates (Bio-Rad, catalog numbers: 1653310 and 1653308 )
Pipette tips (Sarstedt, catalog numbers: 70.760.012, 70.762, 70.3020 and Biozym, catalog number: VZ0001X )
Tris-base (Carl Roth, catalog number: 4855.2 )
Glycine (Carl Roth, catalog number: 00 79.4 )
Colored Prestained Marker (NEB, catalog number: P7719S )
LMW marker (GE Healthcare, catalog number: 17-0446-01 )
Double-distilled water (ddH2O) (Kerndl, catalog number: 22501 )
Rotiphorese (Carl Roth, catalog number: 3029.1 )
Ammonium peroxydisulphate (Carl Roth, catalog number: 9178.1 )
TEMED (Carl Roth, catalog number: 2367.3 )
Glycerol (Carl Roth, catalog number: 3783.2 )
Sodium lauryl sulphate (Carl Roth, catalog number: 4360.2 )
Bromophenol blue (Carl Roth, catalog number: A512.1 )
β-mercaptoethanol (Sigma-Aldrich, catalog number: M6250 )
2-propanol (Carl Roth, catalog number: 6752.5 )
Acetic acid (Carl Roth, catalog number: 6755.1 )
Coomassie blue R 250 (Carl Roth, catalog number: 3862.2 )
Thin-layer chromatography (TLC) of enzymatic assays with radiolabeled nucleotides
Pipette tips (Sarstedt, catalog number: 70.760.012 and Biozym, catalog number: VZ0001X )
Pipette filter tips (Sarstedt, catalog number: 70.1116.210 )
1.5 ml reaction tubes (Sarstedt, catalog number: 72.690.001 )
Thin-layer plate (Macherey-Nagel, POLYGRAM CEL 300 PEI, catalog number: 801053 )
Phosphor imaging plate (Fujifilm, BAS-IP SR 2025 )
Plastic wrap (any type obtained from a grocery store)
Exposure cassette (GE Healthcare, catalog number: 63-0035-44 )
Purified DisA from S. venezuelae or B. subtilis (Witte et al., 2008; Latoscha et al., 2020)
Purified inactive DisA variant (DisAD86A) from S. venezuelae (Latoscha et al., 2020)
Purified AtaC from S. venezuelae (Latoscha et al., 2020)
9.25 MBq [α-32P]-ATP (Hartmann Analytik, catalog number: FP-207 )
9.25 MBq [32P]-c-di-AMP (Hartmann Analytik, catalog number: FP-C517 ; a purified c-di-AMP-producing enzyme, e.g., Bacillus subtilis DisA, must be provided to the company for [32P]-c-di-AMP synthesis upon request)
For phosphodiesterase competition assays:
Magnesium chloride hexahydrate (MgCl2) (Carl Roth, catalog number: 2189.1 )
Manganese (II) chloride tetrahydrate (MnCl2) (Carl Roth, catalog number: T881.1 )
Sodium chloride (NaCl) (VWR, catalog number: 27810.295DB )
Tris-base (Carl Roth, catalog number: 4855.2 )
Hydrochloric acid (Carl Roth, catalog number: 4625.2 )
Glycerol (Carl Roth, catalog number: 3783.2 )
β-mercaptoethanol (Sigma-Aldrich, catalog number: M6250 )
Double-distilled water (ddH2O) (Kerndl, catalog number: 22501 )
0.5 M EDTA, pH 8 (PanReac AppliChem, catalog number: A4892 )
Ammonium sulfate ((NH4)2SO4) (VWR, catalog number: 21333.296DB )
Monopotassium phosphate (KH2PO4) (Carl Roth, catalog number: P018.2 )
DisA cyclase buffer (see Recipes)
PDE reaction buffer (see Recipes)
TLC running buffer (see Recipes)
IEX activity assay
Pipette tips (Sarstedt, catalog numbers: 70.1130.600 and 70.760.502 )
1.5 ml reaction tubes (Sarstedt, catalog number: 72.690.001 )
Microcon-30kDa Centrifugal Filter Unit (Merck Millipore, catalog number: MRCF0R030 )
Purified AtaC from S. venezuelae (Latoscha et al., 2020)
Purified TmPDE from T. maritima (Drexler et al., 2017)
c-di-AMP (BioLog, catalog number: C 088 )
Manganese (II) chloride tetrahydrate (MnCl2) (Carl Roth, catalog number: T881.3 )
Sodium chloride (NaCl) (Merck Millipore, catalog number: 1.06404.5000 )
Tris-base (Carl Roth, catalog number: 4855.2 )
Hydrochloric acid, 25% (VWR, catalog number: 20257.296 )
10x AtaC reaction buffer (see Recipes)
IEX Running buffer A (see Recipes)
IEX Running buffer B (see Recipes)
Equipment
Equipment for buffer exchange (see Notes)
Measuring beaker, 5,000 ml (Carl Roth, catalog number: 0 780.1 )
Magnetic bar (Carl Roth, catalog number: C267.1 )
Pipetting aid macro (Carl Roth, catalog number: X478.1 )
Micropipettes (P20, P200, P1000)
Magnetic stirrer (Heidolph, model: MR 2000 )
Centrifuge (Eppendorf, model: 5427 R, catalog number: 5409000010 )
4 °C room
Equipment for protein concentration determination of your choice (see Notes)
Mini-PROTEAN Tetra Cell (Bio-Rad, catalog number: 1658000EDU )
Heat block (Labnet International, model: AccuBlockTM Digital Dry Bath, catalog number: D1302-230V )
Centrifuge (Eppendorf, model: 5427 R, catalog number: 5409000010 )
Power supply (Bio-Rad, catalog number: 1645050 )
Imager with CCD camera (GE Healthcare, model: ImageQuant LAS 4000 )
Micropipette (P2, P10, P20)
TLC of enzymatic assays with radiolabeled nucleotides
Micropipettes (P2, P10, P20)
Heat block (Labnet International, model: AccuBlockTM Digital Dry Bath, catalog number: D1302-230V )
Centrifuge (Eppendorf, model: 5427 R, catalog number: 5409000010 )
Contamination monitor (Berthold, model: LB 122 )
Biomolecular imager (GE Healthcare, model: Typhoon FLA 7000 , catalog number: 28-9558-09)
Image eraser (Molecular Dynamics, model: 410A )
Acrylic Benchtop Beta Radiation Shield (ThermoFisher Scientific, catalog number: 6700-2418 )
TLC chamber (Fisher Scientific, catalog number: 06-815-187 )
Fume hood
IEX assay
Micropipettes (P2, P20, P200, P1000)
NanoDrop spectrophotometer
Thermomixer (Eppendorf, catalog number: 5382000015 )
Centrifuge (Eppendorf, catalog number: 5401000010 )
Äkta (Cytiva former GE Healthcare)
Resource-Q or Mono-Q (Cytiva, catalog number: 17117701 or 17516601 )
Software
ImageQuantTL (optional) (GE Healthcarre)
Typhoon FLA 7000 control software (GE Healthcare)
PhotoShop CS6 (Adobe)
Unicorn (Cytiva former GE Healthcare)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Latoscha, A., Drexler, D. J., Witte, G. and Tschowri, N. (2021). Assessment of Diadenylate Cyclase and c-di-AMP-phosphodiesterase Activities Using Thin-layer and Ion Exchange Chromatography. Bio-protocol 11(1): e3870. DOI: 10.21769/BioProtoc.3870.
分类
微生物学 > 微生物信号传导 > 第二信使
生物化学 > 蛋白质 > 活性
生物化学 > 其它化合物
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link