- EN - English
- CN - 中文
Establishing an Adult Mouse Brain Hippocampal Organotypic Slice Culture System that Allows for Tracing and Pharmacological Manipulation of ex vivo Neurogenesis
用于追踪和离体神经发生药理学操作的成年小鼠脑器官型海马切片培养系统的建立
发布: 2021年01月05日第11卷第1期 DOI: 10.21769/BioProtoc.3869 浏览次数: 5923
评审: Narayan SubramanianShai BerlinAnonymous reviewer(s)
Abstract
The function of the hippocampus depends on the process of adult hippocampal neurogenesis which underpins the exceptional neural plasticity of this structure, and is also frequently affected in CNS pathologies. Thus, manipulation of this process represents an important therapeutic goal. To identify potential strategies, organotypic adult brain slices are emerging as a valuable tool. Over the recent years, this methodology has been refined and here we present a combined protocol that brings together these refinements to enable long-term culture of adult hippocampal slices. We employ a sectioning technique that retains essential afferent inputs onto the hippocampus as well as serum-free culture conditions, so allowing an extended culture period. To sustain the neurogenic potential in the slices, we utilize the gliogenesis-inhibitor Indomethacin. Using EdU retention analysis enables us to assess the effects of pharmacological intervention on neurogenesis. With these improvements, we have established an easy and reliable method to study the effects of small molecules/drugs on proliferation and neuron formation ex vivo which will facilitate future discovery driven drug screenings.
Keywords: Adult hippocampal neurogenesis (成年海马神经发生)Background
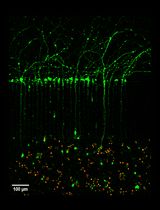
The hippocampus is a unique region of the brain with a high degree of plasticity as a result of the ongoing neurogenesis in the dentate gyrus throughout life. This process of adult hippocampal neurogenesis starts with the asymmetric division of neural stem cells (NSCs) in the subgranular zone (SGZ) that preserves the stem cell pool and generates progenitor cells poised for neuronal differentiation (Kempermann et al., 2004; Anacker and Hen, 2017; Toda and Gage, 2018). These latter cells go through a well-defined sequence of distinct stages: NSC-generated transiently amplifying progenitor cells divide rapidly and give rise to neuroblasts. Neuroblasts are characterized by their expression of the immature neuronal marker Doublecortin (Dcx). They exit the cell cycle to differentiate into immature post-mitotic neurons that in addition to Dcx transiently express the Calcium-binding protein Calretinin as well as the neuronal marker NeuN. In the final step, these newly generated neurons migrate and integrate functionally into the existing neuronal network as mature granule cell neurons which cease to express Dcx and Calretinin, but maintain NeuN expression.
Adult hippocampal neurogenesis plays a fundamental role in physiological CNS functions such as memory consolidation and cognitive flexibility. Thus, not surprisingly, it is implicated in pathologies like Alzheimer’s disease, depression, or schizophrenia (Winner and Winkler, 2015; Anacker and Hen, 2017; Moreno-Jimenez et al., 2019; Park, 2019) and therefore represents an important target for pharmaceutical intervention. However, drug testing is currently often based on cell culture systems that lack the complex architecture and connectivity of the intact brain (Pena, 2010; Humpel, 2015). On the other hand, drug testing in the intact brains of hundreds of animals is not practicable. Organotypic brain slices thus constitute a potential intermediate step as they maintain higher brain cyto-architecture and, as multiple sections can be obtained from one brain, enable comparison of different time points and/or drug concentrations with greatly reduced inter-animal variability. To study the effects of these drugs on neurogenesis, however, a reliable and easy lineage tracing approach is required.
Previously, Kim and colleagues established a protocol to culture adult mouse organotypic hippocampal slices for an extended period of four weeks by employing a serum-free culture medium (Kim et al., 2013). Moreover, they introduced a Hibernate-A based dissection medium as an alternative to more complicated media that required constant gassing with CO2. However, they were unable to trace the fate of single cells, nor did their slices maintain the essential afferent connection to the entorhinal cortex. Kleine Borgmann and co-workers used a different sectioning approach to overcome the latter problem and employed retrovirus labelling to study lineage progression in the SGZ (Kleine Borgmann et al., 2013). Here, we combined and refined these existing organotypic adult slice culture protocols. Moreover, we established EdU label tracing as a suitable read-out for neurogenesis. To circumvent the problem of a decreased neurogenic efficiency following a prolonged culture period, we utilized the Cyclooxygenase-inhibitor Indomethacin that has been shown before to reduce gliogenesis and exert protective effects on neurogenesis in vivo and ex vivo (Gerlach et al., 2016; Hain et al., 2018; Melo-Salas et al., 2018). Finally, establishing proof of concept as to the value of this protocol we compared the effects of Silychristin, iopanoic acid, and BCH on neurogenesis. These drugs are small molecular inhibitors of the monocarboxylate transporter 8 (Mct8), deiodinase type 2 (Dio2), and L-type amino acid transporters 1/2 (Lat 1/2) respectively–components of central thyroid hormone signalling. While neither iopanoic acid nor BCH treatment altered ex vivo neurogenesis, the number of new neurons was significantly reduced in Silychristin-treated cultures (Mayerl et al., 2020).
Taken together, these improvements enabled us to establish a method that allows for i) an extended culture period of adult brain slices for at least three weeks with good neurogenic efficiency, ii) easy lineage tracing using EdU that also provides a defined and controlled starting point, and iii) pharmacological manipulation of ex vivo hippocampal neurogenesis. We believe that our advanced methodology will be useful for future drug screening approaches to sustain or improve hippocampal neurogenesis in various pathological conditions or physiological alterations such as ageing.
Materials and Reagents
10 cm Petri dish (Thermo Fisher Scientific, Corning, catalog number: BP94A01 )
6-well-plate (Corning, catalog number: 3516 )
12-well-plate (Corning, catalog number: 3513 )
Tin foil
Microscope slides SuperFrost PlusTM (Thermo Fisher Scientific, catalog number: 10149870 )
Coverslips (e.g., 24 x 50 mm) (Thermo Scientific Menzel x1000 Coverslip 24 x 50 mm #1, catalog number: 15737592 )
Peel-A-Way® 22 x 40 mm x 20 mm deep, rectangular embedding molds (Ted Pella, catalog number: 27114 )
Millicell Cell Culture Insert, 30 mm, hydrophilic PTFE, 0.4 µm pore size (Merck, catalog number: PICMORG50 ), shelf storage
Mice (e.g., C57/Bl6N)
EdU (5-ethynyl-2-deoxyuridine) (Thermo Fisher Scientific, InvitrogenTM, catalog number: A10044 (50 mg), stored in 500 µl aliquots at -20 °C
HibernateTM-A Medium (Thermo Fisher Scientific, GibcoTM, catalog number: A1247501 ), stored at 4 °C for a maximum of 4 months
B-27TM supplement (50x) (Thermo Fisher Scientific, GibcoTM, catalog number: 17504001 ), aliquots stored at -20 °C, after defrosting stored at 4 °C for a maximum of 1 month
Agarose, low melting point (Merck, Calbiochem, catalog number: 2070-100GM ). 4% solution in PBS can be stored on shelf for up to 6 months and re-used 3-4x
NeurobasalTM-A Medium (Thermo Fisher Scientific, GibcoTM, catalog number: 10888022 ), stored at 4 °C for a maximum of 4 months
Indomethacin (Merck, Sigma-Aldrich, catalog number: I7378-5G ), stored at 4 °C as stock solution of 100 mM in DMSO for a maximum of 4 months
Silychristin (Merck, Sigma-Aldrich, catalog number: 51681-10MG ), stored at 4 °C as a stock solution of 100 mM in DMSO for a maximum of 4 months
Iopanoic acid (Merck, Sigma-Aldrich, catalog number: I0330000 ), stored at 4 °C as a stock solution of 100 mM in DMSO for a maximum of 4 months
BCH (R&D Systems, catalog number: 5027/50 ), stored at 4 °C as a stock solution of 100 mM in Neurobasal A for a maximum of 4 months
Formaldehyde 37% (Merck, Sigma-Aldrich, catalog number: 252549-500ML ), stored in a ventilated fume hood
Click-iT® EdU Alexa Fluor® 647 Imaging Kit (Thermo Fisher Scientific, Molecular Probes, catalog number: C10340 ), components diluted and stored according to manufacturer’s instructions
Rabbit-anti-Ki67 antibody (Abcam, catalog number: ab16667 ), aliquots stored at -20 °C
Guinea pig-anti-Doublecortin (Dcx) antibody (Merck, Millipore, catalog number: ab2253 ), aliquots stored at -20 °C
Mouse-anti-NeuN antibody (Merck, Millipore, catalog number: mab377 ), stored at 4 °C
Fluoromount-G® (Southern Biotech, catalog number: 0100-01 ), stored at room temperature
Super glue
Triton X-100 (Merck, Sigma-Aldrich, catalog number: X100-1L ), stored at room temperature
Glycine (Merck, Sigma-Aldrich, catalog number: G7126-1KG ), stored at room temperature
10x PBS (Thermo Fisher Scientific, Life Technologies, catalog number: 70011-036 500ml), stored at room temperature; dilute with distilled water
BSA (Merck, Sigma-Aldrich, catalog number: A7906-50G ), stored at 4 °C
Goat serum (Merck, Sigma-Aldrich, catalog number: G6767-100ML ), stored at -20 °C
Hoechst33258 (Thermo Fisher Scientific, Life Technologies, catalog number: 62249 5ML ), stored at 4 °C
L-Glutamine (Invitrogen, catalog number: 25030-024 ), aliquots stored at -20 °C
Penicillin/Streptomycin (10,000 U/ml Pen; 10,000 µg/ml Strep; Invitrogen, catalog number: 15140-122 ), aliquots stored at -20 °C
DMSO (Thermo Fisher Scientific, catalog number: D12345 3ML ), stored at room temperature
7 ml tubes (Sarstedt, Tube 7ml 47x20PC+Cap, catalog number: 71.9923.610 ), stored at room temperature
Dissection buffer (see Recipes)
Serum-free slice culture medium (see Recipes)
Equipment
Pipettes
Brush
Spatula (Thermo Fisher Scientific, FisherbrandTM Nickel Chattaway Spatula, catalog number: 11583462 "> 11583462 )
Scalpel (disposable scalpel, Swann Morton No. 11, Medisave, catalog number: SKU0503 )
Tissue culture hood
Water bath
Forceps (Fine Science Tools, Dumont #5 Forceps Standard Carbon, catalog number: 11251-10 )
Scissors (Fine Science Tools, Fine Scissors, Sharp, catalog number: 14060-10 )
Rocker
Confocal microscope (Leica SP8 )
Vibratome (Leica VT1000 S )
Software
ImageJ (NIH, https://imagej.nih.gov/ij/)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Mayerl, S. and ffrench-Constant, C. (2021). Establishing an Adult Mouse Brain Hippocampal Organotypic Slice Culture System that Allows for Tracing and Pharmacological Manipulation of ex vivo Neurogenesis. Bio-protocol 11(1): e3869. DOI: 10.21769/BioProtoc.3869.
分类
神经科学 > 细胞机理 > 细胞分离和培养
干细胞 > 成体干细胞
细胞生物学 > 基于细胞的分析方法 > 药物筛选
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link