- EN - English
- CN - 中文
Double Labeling of PDGFR-β and α-SMA in Swine Models of Acute Kidney Injury to Detect Pericyte-to-Myofibroblast Transdifferentation as Early Marker of Fibrosis
双标记PDGFR-β和α-SMA在猪急性肾损伤模型中检测周细胞-肌成纤维细胞转化作为早期纤维化标志物的研究
(*contributed equally to this work) 发布: 2020年10月05日第10卷第19期 DOI: 10.21769/BioProtoc.3779 浏览次数: 4341
评审: Anonymous reviewer(s)
Abstract
Growing evidences suggest that peritubular capillaries pericytes are the main source of scar-forming myofibroblasts during chronic kidney disease (CKD), as well as early phases of acute kidney injury (AKI). In a swine model of sepsis and I/R (Ischemia Reperfusion) injury-induced AKI we demonstrated that renal pericytes are able to transdifferentiate toward α-SMA+ myofibroblasts leading to interstitial fibrosis. Even if precise pericytes identification requires transmission electron microscopy and the co-immunostaining of several markers (i.e., Gli, NG2 chondroitin sulphate proteoglycan, CD146, desmin or CD73) and emerging new markers (CD248 or TEM1, endosialin), previous studies suggested that PDGFR-β could be used as marker for renal pericytes characterization. Recently, double immunofluorescence staining of PDGFR-β and α-SMA was performed to identify the damage activated pericytes (PDGFR-β+/α-SMA+ cells) in the early phase of fibrosis development. Our data highlighted the crucial role of renal pericytes in the physiopathology of sepsis and I/R associated AKI. In this protocol, we describe the procedure for double immunofluorescence staining of PDGFR-β and α-SMA in swine Formalin-Fixed Paraffin-Embedded (FFPE) kidney biopsies and the method for image analysis and quantification.
Keywords: PDGFR-β+/α-SMA+ immunofluorescence, (PDGFR-β+/α-SMA+ 荧光免疫检验法)Background
Renal fibrosis is considered the principal responsible for progression of renal disease and it is associated with a limited capacity of kidney to regenerate after injury. The principal source of interstitial fibrosis in progressive renal diseases (Simone et al., 2014; Fiorentino et al., 2018) is represented by activated fibroblasts, named myofibroblasts, that are recognized by their ability to synthesize de novo α-smooth muscle actin (α-SMA) (Meran and Steadman, 2011; Hewitson, 2012). These cells derived from multiple sources, including not only resident fibroblasts, but also endothelial cells, tubular cells, circulating bone marrow–derived cells and pericytes (Di Carlo and Peduto, 2018). Several studies evaluated the critical role of capillary pericytes in chronic kidney disease (CKD) (Lin et al., 2008; Grgic et al., 2012; Kramann et al., 2015) as well as early phases of acute kidney injury (AKI) (Leaf et al., 2016; Castellano et al., 2018 and 2019).
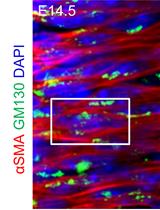
Recent studies revealed that dysfunctional pericytes are principally involved in sepsis-induced microvascular dysfunction and vascular leakage which are the key hallmarks of end-organ dysfunction and septic shock (Goldenberg et al., 2011; Page and Liles, 2013; Castellano et al., 2014; Stasi et al., 2017; Fani et al., 2018). Consistent with these findings, we found that pigs challenged with LPS led to a dysfunctional response of pericytes in the microvasculature of renal parenchyma (Castellano et al., 2019) (Figure 1).
In recent years, the fate-tracing mapping and the ultrastructural analysis have shed more lights on the pericytes behavior in several mouse model of renal diseases (Lin et al., 2008; Humphreys et al., 2010). In fairness, the identification of pericytes by criteria that requires elaborate techniques as fate-tracing analysis is not practical in large animal model. Since PDGFR-β is defined as the constitutive marker for isolation and characterization of renal pericytes (Chen et al., 2011; Wang et al., 2017) and a-SMA is a marker associated with myofibroblasts (Hewitson, 2012), the double-labeling for PDGFR-β and α-SMA provide a “picture” of pericyte distribution and dysfunctional activation in renal parenchyma during AKI (Guzzi et al., 2019). In accordance with other phenomena of cellular transdifferentation as EndMT, the loss of PDGFR-β and the increased level of α-SMA, Collagen I and MMP proteins is defined as Pericytes to Myofibroblast transition (PMT) (Chang et al., 2012).
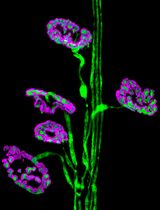
We, firstly, performed double immunofluorescent staining of PDGFR-β and α–SMA to study the PMT in swine and mice models of renal I/R injury (Figure 2) (Castellano et al., 2018). Interestingly, in normal swine kidney biopsies we found the colocalization of the PDGFR-β and NG2 in peritubular capillaries, indicating the overall accuracy of PDGFR-β as capillary pericytes markers in pig models whereas glomerular mesangial cells were PDGFRβ+ but NG2−. After 24h of renal I/R we observed the downregulation of the constitute markers PDGFR-β and NG2 and the dramatic upregulation of α-SMA.
These data were also confirmed in our sepsis model of AKI and taken together we demonstrated that PDGFR-β+pericytes are able to synthesize pro-fibrotic markers acquiring the typical features of myofibroblasts, leading to extracellular matrix deposition and interstitial fibrosis (Castellano et al., 2019). In accordance, we confirmed our data in vitro and we found that exposition of human placental derived pericytes to I/R injury (C5a) and sepsis stimuli (LPS) led to acquisition of α-SMA contractile stress fibers (Castellano et al., 2018 and 2019).
Therefore, the protocol described here could be useful to characterize pericytes and their dysfunctional activation and will facilitate the research in the early acute kidney injury as well as other fields relating inflammation and fibrosis (Castellano et al., 2018 and 2019).
Materials and Reagents
- Aluminium Foil (Carl Roth GmbH, Rotilabo®, catalog number: 0 954.1 )
- StripetteTM Serological Pipets 5 ml, 10 ml (Corning, catalog numbers: 4051 , 4488)
- Dish 60 mm (Corning, catalog number: 3261 )
- Falcon® centrifuge tube 50 ml,15 ml (Corning, catalog numbers: 352070 , 352096)
- Safety safeshield scalpels (Biosigma srl, catalog number: 530050 )
- Metal steel base mold (Leica Biosystem, catalog number: 3803081 )
- Coverslips for optical microscopy 24 x 40 x 0.16 mm (Bio Optica SpA, catalog number: 09-2040 )
- Paper towel
- Tissue Embedding Rings (Bio Optica SpA, catalog number: 07-7650 )
- Nail varnish
- 10% neutral buffered formalin (NBF) (Diapath SpA, catalog number: F0047)
- Paraffin Lab O-Wax 56-58 °C (Histo-Line Laboratories srl, catalog number: R0040-20 )
- Sterile DPBS (Euroclone SpA, catalog number: ECM4053XL )
- Xylene (Bio Optica SpA, catalog number: 06-1304Q )
- Ethanol absolute (100%) (Bio Optica SpA, catalog number: 06-10099 )
- Deionized water
- Microscopy slides Super Frost® Plus (Bio Optica SpA, catalog number: 09-OPLUS )
- Normal Goat Serum (Sigma-Aldrich, catalog number: G9023 , stored in aliquots at -20 °C)
- Phosphate Buffered Saline Tablets (Sigma-Aldrich, catalog number: P4417 )
- α-SMA mouse monoclonal (Santa Cruz Biotechnology, catalog number: sc-32251 )
- PDGFR-β monoclonal antibody rabbit anti human (Abcam, catalog number: ab32570 )
- AlexaFluor goat anti-rabbit FITC-conjugated (488 nm) antibody (Molecular Probes, catalog number: A11070 )
- AlexaFluor goat anti-mouse TRITC conjugated (555 nm) antibody (Molecular Probes, catalog number: A21127 )
- TO-PRO3 IODIDE (Invitrogen, catalog number: T3605 )
- FluoroMount (Bio-Optica SpA, catalog number: K024 )
- Phosphate buffered saline tablet (Sigma, catalog number: P4417 )
- Acid citric (Sigma, catalog number: C759 )
- Sodium citrate (Sigma, catalog number S4641)
- Normal Goat Serum (Sigma-Aldrich, catalog number G9023 , stored in aliquots at -20 °C)
- Unmasking Buffer (10 mM Sodium Citrate Buffer) (see Recipes)
- Blocking solution (10% Normal Goat Serum) (see Recipes)
- Buffer for diluting primary antibody (5% Normal Goat Serum) (see Recipes)
- PBS 1x for the IF washing (see Recipes)
Equipment
- Autoclave (AHSI S.p.A., model: FVA/A1 )
- Tweezers (Thermo Fisher Scientific, catalog number: 10303611 )
- Scissors for microscopy (Bio Optica SpA, catalog number: 32-703 )
- Gloves and Eye protection
- Hot plate with magnetic stirrer
- PMP Hellendhal Staining Jar (Kartell S.p.A., LABWARE Division, KartellTM, catalog number: 00 35500 )
- Water bath
- Fume cupboard (Euroclone SpA)
- Pipettes (0.5-10, 10-100 and 100-1,000 μl) (Eppendorf, catalog numbers: 3111000.122 , 3111000.149 , 3111000.165 )
- Bard monopty biopsy instrument 16 G x 20 cm length (C.R. Bard.Inc., catalog number: 121620 )
- 58 °C paraffin bath Cold plate (Bio Optica SpA, catalog number: PF100 )
- Microtome (Leica Biosystem, catalog number: RM2125 RTS )
- Histology Bath (Bio Optica SpA, catalog number: WB100 )
- Microwave
- Pap pen for immunostaining (Bio Optica SpA, catalog number: 11-100 )
- Orbital shaker (Thermo Scientific, catalog number: SHKA2000 )
- Humidified Chamber
- pH meter (Thermo Scientific, catalog number: 3115101 )
Software
- GraphPad Prism® (version 5.0) (GraphPad Software)
- Image analysis software (Leica QWin) (Leica)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Stasi, A., Franzin, R., Divella, C., Gesualdo, L., Stallone, G. and Castellano, G. (2020). Double Labeling of PDGFR-β and α-SMA in Swine Models of Acute Kidney Injury to Detect Pericyte-to-Myofibroblast Transdifferentation as Early Marker of Fibrosis. Bio-protocol 10(19): e3779. DOI: 10.21769/BioProtoc.3779.
分类
细胞生物学 > 细胞染色 > 其它化合物
细胞生物学 > 组织分析 > 组织染色
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link