- EN - English
- CN - 中文
Fluorescence Measurement and Calibration of Intracellular pH in Starfish Oocytes
海星卵母细胞内pH值的荧光测定与校准
发布: 2020年10月05日第10卷第19期 DOI: 10.21769/BioProtoc.3778 浏览次数: 4515
评审: Imre GáspárAbhijit KaleAnonymous reviewer(s)
Abstract
Oocyte maturation is a process wherein an oocyte arrested at prophase of meiosis I resumes meiosis to become a fertilizable egg. In starfish ovaries, a hormone released from follicle cells activates the oocytes, resulting in an increase in their intracellular pH (pHi), which is required for spindle assembly. Herein, we describe a protocol for pHi measurement in living oocytes microinjected with the pH-sensitive dye BCECF. For in vivo BCECF calibration, we treated oocytes with artificial seawater containing CH3COONH4 to clamp pHi, injected pH-standard solutions, and converted the BCECF fluorescence intensity ratios to pHi values. Of note, if the actual pHi is higher or lower than the known pH of injected standard solutions, the BCECF fluorescence intensity ratio will decrease or increase, respectively. On the other hand, the pH of the injected solution displaying no change in fluorescence intensity should be considered the actual pHi. These methods for pHi calibration and clamping are simple and reproducible.
Keywords: Intracellular pH (细胞内pH值)Background
Intracellular pH (pHi) measurement is an extremely useful method for the study of cell biology since pHi plays important roles in a variety of cell processes, such as gametes’ activation (Johnson and Epel, 1976; Shen and Steinhardt, 1978; Tilney et al., 1978), cell division (Schuldiner and Rozengurt, 1982; Moolenaar et al., 1983; Anand and Prasad, 1989; Karagiannis and Young, 2001), and cancer cell survival (Grillo-Hill et al., 2015).
An oocyte at prophase, during meiosis I, is quiescent until hormonal stimulation resume meiosis. Studies on starfish oocytes have reported that the hormone 1-methyladenine (1-MA) binds to an unidentified receptor on the plasma membrane of oocytes (Tadenuma et al., 1992), dissociating the heterotrimeric GTP-binding protein (G) α-subunit (Gα) from the βγ subunit (Gβγ), which activates phosphatidylinositol-3 kinase (PI3K) (Chiba et al., 1993; Sadler and Ruderman, 1998). Thereafter, serum- and glucocorticoid-regulated kinase (SGK) is phosphorylated and activated by the target of rapamycin complex2 (TORC2) and 3-phosphoinositide-dependent protein kinase 1 (PDK1) (Hiraoka et al., 2019; Hosoda et al., 2019). Starfish SGK is required for Na+/H+ exchanger (NHE) dependent pHi increase from ~6.7 to ~6.9 in the ovarian oocytes (Harada et al., 2010; Moriwaki et al., 2013; Hosoda et al., 2019). Simultaneously, SGK phosphorylates Cdc25 and Myt1, inducing the de-phosphorylation and activation of cyclin B–Cdk1, causing germinal vesicle breakdown (GVBD) (Hiraoka et al., 2019). Importantly, both pHi increase and GVBD are required for spindle assembly of ovarian oocytes at metaphase I (Harada et al., 2003; Hosoda et al., 2019), followed by MI arrest at pHi 6.9 until spawning (Moriwaki et al., 2013).
Fluorescence indicators, including 2',7'-bis(carboxyethyl)-5,6-carboxyfluorescein (BCECF) have been used to measure pHi (Rink et al., 1982; Bassil et al., 2013; Behbahan et al., 2014; Mortera et al., 2015). BCECF is a pH-sensitive and ratiometric dye requiring dual-excitation. To calibrate pHi in vivo, BCECF-loaded cells are treated with buffer containing the K+/H+ exchanger nigericin and K+ at high concentration since pHi is expected to become equal to the extracellular pH, adjusted to known values in the presence of nigericin, if extracellular K+ is equal to intracellular K+ (Thomas et al., 1979; Rink et al., 1982). However, it is difficult to use this method when the intracellular K+ concentration is unknown. In another study, in vitro BCECF calibration without cells was performed to estimate pHi; however, the pKa of intracellular BCECF is sometimes greater than that of the in vitro solution (Boyarsky et al., 1996). Permeabilization of the cell membrane with digitonin or Triton-X-100 is another method used for pHi calibration (Rink et al., 1982); however, the fluorescence ratio is often unstable owing to cell lysis (Harada et al., 2003; Moriwaki et al., 2013).
Weak bases and acids can directly affect pHi since all membranes are permeable to uncharged molecules (Roos and Boron, 1981). For example, NH4Cl in seawater forms NH4+ and NH3, and the uncharged NH3 penetrates the cell membrane and binds to intracellular H+, thus increasing the pHi. In contrast, CH3COONa dissolved in seawater forms CH3COO- and CH3COOH; thereafter, CH3COOH penetrates the cell membrane, releasing H+ and decreasing pHi (Hamaguchi et al., 1997). Similarly, CH3COONH4 dissolved in seawater forms NH4+, NH3, CH3COO-, and CH3COOH. NH3 and CH3COOH easily penetrate the cell membrane, and bind to or release H+, respectively. Of note, in seawater at a higher pH, the NH3 concentration is greater than that of CH3COOH, resulting in a higher NH3 concentration and an increase in pHi by forming NH4+. In contrast, at a lower pH, the seawater-derived intracellular CH3COOH concentration is higher than that of NH3, thus decreasing the pHi (Figure 1). Moreover, the increase in NHE-dependent pHi is inhibited in sodium-free artificial seawater. In fact, using sodium-free artificial seawater (ASW) containing CH3COONH4 (modified ASW) at various pH values, we were previously able to clamp the pHi of starfish oocytes at desired pH values (Moriwaki et al., 2013; Hosoda et al., 2019).
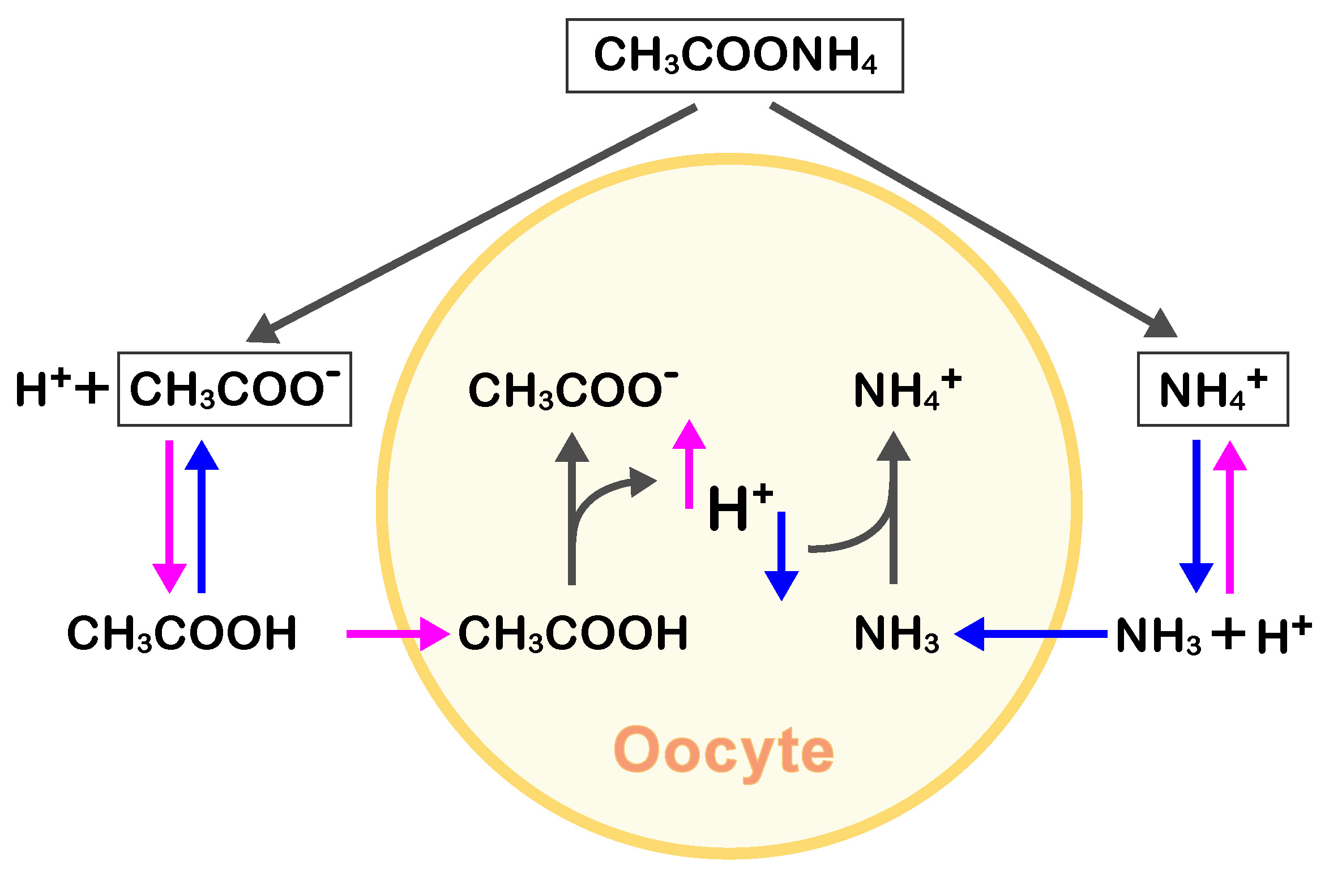
Figure 1. Diagram showing the CH3COONH4-based equilibrium in seawater and its effect on intracellular pH. In seawater with a higher pH, the NH3 concentration is greater than that of CH3COOH (blue arrows) due to equilibrium shift, resulting in a higher intracellular NH3 concentration in oocytes, permeable to the uncharged form of NH3. Then, intracellular NH3 in oocytes increases pHi via NH4+ formation. Conversely, at a lower seawater pH, the intracellular uncharged CH3COOH concentration is higher than that of NH3, thus decreasing the pHi (magenta arrows).
Furthermore, we could estimate the actual pHi of oocytes in modified ASW via the injection of standard solutions. When the actual (or real) pHi is higher than the known pH of the injected standard solution, the BCECF fluorescence intensity ratio decreases since the pHi is also decreased upon injection of standard solutions at lower pH values. On the other hand, when the actual pHi is lower than the known pH of the injected standard solutions, the abovementioned ratio increases. Thus, the actual pHi should be between these two pH values. Indeed, based on this premise, we were able to select the injection solutions at the actual pHi, resulting in no change in the intensity ratio upon their administration (Moriwaki et al., 2013; Hosoda et al., 2019).
Here, to calibrate the pHi of oocytes stimulated with 1-MA in normal SW, we treated immature oocytes with modified ASW, clamping pHi at higher and lower values, and thus yielding high and low BCECF fluorescence intensity ratios. Thereafter, we injected these oocytes with the standard solutions to estimate the actual pHi values. Using these references, we conducted a two-point calibration, forming a straight line crossing the two points. Although a calibration using ≥ 3 points is achievable, we confirmed that the two-points’ calibration graph was linear–as per the calibration data obtained using more than three points (Moriwaki et al., 2013; Hosoda et al., 2019). Thus, we could convert the fluorescence ratios of maturing oocytes in normal seawater to pHi, using this standard linear calibration graph/function-based method (Moriwaki et al., 2013; Hosoda et al., 2019).
Materials and Reagents
- Glass capillary for a glass micropipette, Microcap, 50 μl (Drummond Scientific Company, catalog number: 1-000-0500)
A micropipette was made using the puller PC-100 system, as per the manufacturer’s instructions (Narishige, https://products.narishige-group.com/group1/PC-100/pipette/english.html). The properties (length + outer/inner diameter) of the ideal capillaries are shown in Figure 2A. To control precisely the flow volume out of the glass micropipette, a constriction was made using a handmade loop of platinum wire. Briefly, the micropipette was passed through the loop of the platinum wire. Then, the micropipette (3-5 mm from the end) was heated until a constriction of 3.5-5.0 µm in diameter was formed via the application of an electric current to the loop (see also Hiramoto, 1974). - Cover glasses (Matsunami, 18 mm x 18 mm, thickness 0.12-0.17 mm)
- Silicone oil (Shin-Etsu Chemical Co.Ltd. catalog number: KF-96-100CS ) (Figure 2C)
- A dextran (10-kD) BCECF conjugate (Fisher Scientific, InvitrogenTM, catalog number: D1878 ) (Figure 2B)
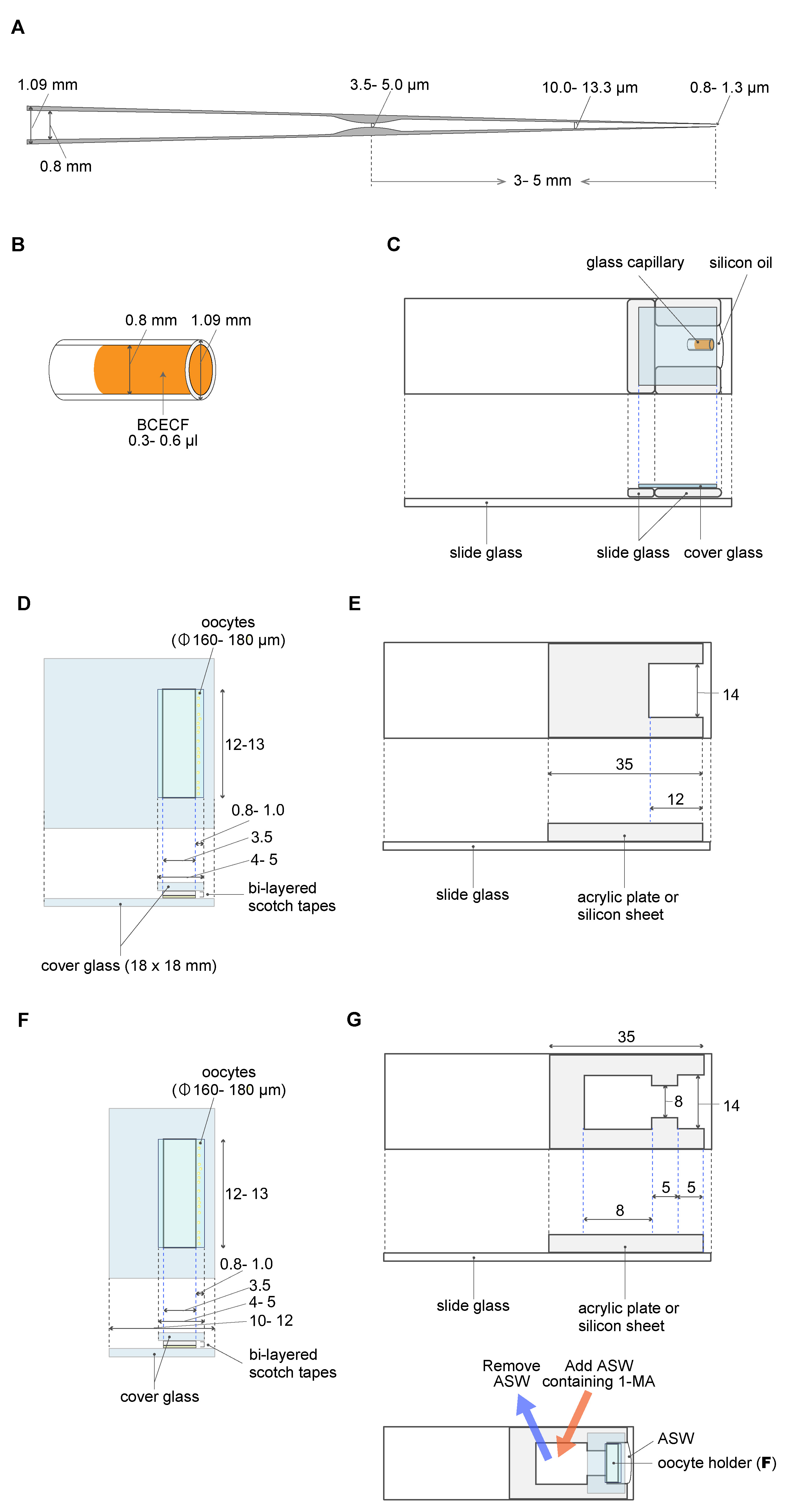
Figure 2. Materials and equipment used for oocyte manipulation. A. The constriction made in a glass micropipette acts as a brake. B. BCECF solution in a glass capillary. C. BCECF solution in a glass capillary is set in the capillary holding chamber filled with silicone oil. The glass micropipette (A) filled with silicone oil is inserted into the BCECF solution; 20 pL BCECF is aspirated into the micropipette. D. The oocyte holder. E. The manipulation chamber. F. The oocyte holder for 1-MA treatment. G. The manipulation chamber for 1-MA treatment. The chamber has an opening that allows the removal of an ASW volume (25%) and its replacement by the same volume of ASW containing 1-MA. D-G. All measurements are represented in mm unless otherwise indicated. - 1-methyladenine (KANTO CHEMICAL CO. INC., catalog number: 20131-1A )
- Potassium aspartate (Tokyo Chemical Industry Co., Ltd., catalog number: A0922 )
- HEPES, 2-[4-(2-Hydroxyethyl)-1-piperazinyl] ethanesulfonic acid (DOJINDO, catalog number: 340-01371 )
- Pipes, Piperazine-1,4-bis (2-ethanesulfonic acid) (DOJINDO, catalog number: 347-02224 )
- KOH (Wako Pure Chemical Industries, Ltd., catalog number: 168-21815)
- NP-40 (Nonidet P-40. Nacalai Tesque, catalog number: 252-23 )
- NaCl (FUJIFILM Wako Pure Chemical Corporation, catalog number: 191-01665 )
- KCl (KANTO CHEMICAL CO. INC., catalog number: 32326-00 )
- MgCl2•7H2O (Wako Pure Chemical Industries, Ltd., catalog number: 135-00165 )
- MgSO4•7H2O (Wako Pure Chemical Industries, Ltd., catalog number: 131-00405 )
- H3BO3 (Showa Chemical Co. Ltd., catalog number: 0214-6250 )
- CH3COONH4 (Wako Pure Chemical Industries, Ltd., catalog number: 019-02835 )
- CaCl2•2H2O (Wako Pure Chemical Industries, Ltd., catalog number: 031-00435 )
- Calcium-free ASW (see Recipes)
- ASW (see Recipes)
- Modified ASW pH 6.8 (see Recipes)
- Standard pH solutions at pH 6.40-6.75 or 6.80-7.40 (see Recipes)
- BCECF solution (see Recipes
Equipment
- CMOS camera (Hamamatsu Photonics K.K., ORCA-Flash2.8, model: C11440-10C )
- ECLIPSE Ti-U fluorescent microscope (Nikon Instech)
- 4× 0.20 NA CFI super Fluor lens (Nikon Instech)
- Navi h pH meter (HORIBA, pH METER, F-52)
- Screw-controlled microinjector (Narishige, model: IM-9B )
Software
- HCImage U11158-02, 03 (Hamamatsu Photonics K.K., https://hcimage.com/)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Hosoda, E. and Chiba, K. (2020). Fluorescence Measurement and Calibration of Intracellular pH in Starfish Oocytes. Bio-protocol 10(19): e3778. DOI: 10.21769/BioProtoc.3778.
- Hosoda, E., Hiraoka, D., Hirohashi, N., Omi, S., Kishimoto, T. and Chiba, K. (2019). SGK regulates pH increase and cyclin B-Cdk1 activation to resume meiosis in starfish ovarian oocytes. J Cell Biol 218(11): 3612-3629.
分类
发育生物学 > 细胞生长和命运决定 > 卵母细胞
细胞生物学 > 细胞成像 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link