- EN - English
- CN - 中文
A Method to Efficiently Cryopreserve Mammalian Cells on Paper Platforms
一种纸质平台高效冷冻保存哺乳动物细胞的方法
(*contributed equally to this work) 发布: 2020年09月20日第10卷第18期 DOI: 10.21769/BioProtoc.3764 浏览次数: 4784
评审: Alessandro DidonnaPreeti SharmaAnonymous reviewer(s)
Abstract
This protocol describes a simple method to cryopreserve mammalian cells within filter papers as an alternative to conventional slow-freezing approach. The method involves treating paper fibers with fibronectin, using low concentrations of the cryoprotectant dimethyl sulfoxide (DMSO), and slow freezing cells to -80 °C at a 1 °C min-1 rate. In our method, the biocompatibility, large surface area, 3D porosity and fiber flexibility of the paper, in combination with the fibronectin treatment, yield recovery of cells comparable to conventional approaches, with no additional fine-tuning to freezing and thawing procedures. We expect that the paper-based cryopreservation method will bring several advantages to the field of preserving mammalian cells, including accommodation of a higher number of cells within a unit volume and no cell loss after release. The method requires a minimal storage space, where paper platforms with large areas can be rolled and/or folded and stored in stocks, and allows for efficient transportation/distribution of cells in an on-demand manner. Moreover, an additional feature of this method includes the formation and cryopreservation of cellular spheroids and 3D cell cultures.
Keywords: Paper (纸)Background
Successful preservation, long-term storage, maintenance and distribution of mammalian cells are important research areas that are still under intense scientific investigation. In particular, the timely and steady supply of frozen cells is pertinent in tissue engineering research such as cell culture, drug development and testing, and regenerative and biotherapeutic medicine.
Current conventional cell cryopreservation protocols include slow and rapid freezing and vitrification (Pegg, 2002; Baust et al., 2009). In these approaches, cryoprotectant agents at various concentrations are added to the cell suspension, followed by cooling down the medium at temperature rates as low as 1 °C min-1 (slow freezing) to as high as 120 °C min-1 (rapid freezing), or by placing samples directly in -195 °C liquid nitrogen tanks (vitrification). As a result, with the protective role of cryoprotectants, the cell damage or death is minimized during freezing (Karlsson and Toner, 1996; Asghar et al., 2014; Jang et al., 2017). However, these approaches possess limitations in terms of requiring large storage spaces to house the cells in thousands to millions of small cryotubes and cryobags (Heidemann et al., 2010; Massie et al., 2014). The distribution of cells then faces challenges in terms of loss, misidentification, and logistics management (Tomlinson, 2005).
Recent approaches described the use of various engineered porous scaffolds for cryopreservation of cell tissue constructs. Examples include the use of corn starch-polycaprolactone fiber meshes (Costa et al., 2012), electrospun-polyurethane nanofiber sheets (Batnyam et al., 2017), alginate-gelatin cryogel sponges (Katsen-Globa et al., 2014), and reticulated polyvinyl formal resins (Miyoshi et al., 2010). The results of these studies proved that the biocompatibility, mechanical support, and 3D porosity of scaffolds provide a suitable balance in creating a protective microenvironment for the cells during their cryopreservation. However, with these scaffolds, substrates need to be repeatedly manufactured (i.e., engineered) for their use in relevance to cryopreservation of cells, which brings a heavy dose of hurdle to the field.
Remarkably, paper-based platforms have emerged as an attractive alternative for tissue engineering development, and especially for 3D cell culture. With added features such as cost-effectiveness and tunable fiber surface characteristics, these ready-to-use scaffolds offer remarkable applicability for large-scale, multilayer biological testing (Derda et al., 2009; Mosadegh et al., 2014). As a result, various cellular applications utilizing paper platforms have been intensively investigated (Ng et al., 2017; Wu et al., 2018; Rosqvist et al., 2020). Yet, despite its outstanding potential as a substrate for 3D cell culture and molecular sampling, paper platform has never been utilized to its full potential in directly cryopreserving cells, until our recent work (Alnemari et al., 2020). Instead, it was used as a vitrification container (2D paper substrate) to enhance cryopreservation of mouse embryos (Paul et al., 2018), bovine oocytes (Kim et al., 2012), and bovine blastocysts (Lee et al., 2013). FTA cards, on the other hand, were used for the collection, storage (at room temperature or at +4 °C, -20 °C and -80 °C), transportation, and molecular analysis of nucleic acids (Santos, 2018).
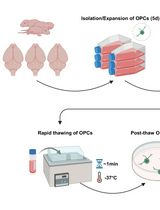
In this protocol, we give step-by-step explanations (Figure 1) on how to 3D preserve and release mammalian cells using our developed paper-based cryopreservation method (Alnemari et al., 2020). The technique starts with cutting the filter paper into small strips (e.g., 3 × 3 cm2). Then, paper fibers are treated with fibronectin to enhance the post-thawing cell release. This is trailed by suspending cells in serum medium containing low concentration dimethyl sulfoxide (DMSO). Following, cell suspension is pipetted onto fibronectin-treated papers. Immediately after cells penetrate within the paper’s 3D porous matrix, papers are rolled and placed in standard cryotubes, slow freezed to -80 °C at a 1 °C min-1 rate, and kept in -195 °C liquid nitrogen for long-term storage. The cells can then be thawed and, depending on the need, either released from the paper to expand as typical 2D culture in flasks or kept inside the paper to grow as 3D cultures and spheroids.
In the developed method, cells are ubiquitous in the 3D porous environment of the paper, where paper fibers provide a natural protective and supportive environment during their cryopreservation. As a result, after their freeze, thawed cells are efficiently released from paper with high viability rates by gently shaking the paper. Here, any paper type, with pores suitable for cells to penetrate within, can be used by simply optimizing the fibronectin concentration for the effective release of cells. The paper also provides a versatile environment for the remaining cells within the paper to grow as aggregates (spheroids) and, as well, successfully enables the formation (by using Matrigel matrix) and cryopreservation of 3D cell cultures (Alnemari et al., 2020). The paper-based cryopreservation offers space-saving and efficient cell transportation/distribution solutions in a cost-effective, fast and easy-to-manage manner, since large paper sheets can be rolled and/or folded to fit standard cryotubes (or other contianers) and stored in stocks and cut into small pieces without the need to thaw the entire platform.
Figure 1. Paper-based cell cryopreservation method. A. An outline of stages involved in the paper-based cryopreservation method. After the thaw, cells can be either released from paper by gentle shaking and expand as 2D culture in flasks or 3D cultured in vitro as needed. B. Micrographs visualize the way a 3 × 3 cm2 paper strip is treated and placed in a cryotube after rolling.
Materials and Reagents
The materials and reagents presented below are for paper-based cryopreservation of cervical HeLa cell lines. For cryopreservation of breast MCF-7, prostate PC3 and lymphocyte JKT cell lines, see the section “Notes” for specific details.
Required
- Sterile 10 ml serological pipettes (Costar Stripette, catalog number: 4488 )
- Sterile 15 ml centrifuge tubes (ThermoFisher, catalog number: 339650 )
- Sterile 1-20 μl, 200 μl, 1,000 μl pipette tips (ThermoFisher, catalog numbers: 10380792 , 10619331 , 10390792 )
- Sterile T75 tissue culture flasks (ThermoFisher, catalog number: 156499 )
- Sterile 1 ml cryogenic tubes (cryotubes) (Sigma-Aldrich, catalog number: CLS430487 )
- Cryo-safe vial storage boxes (cryoboxes) (Sigma-Aldrich, catalog number: Z756776 )
- Mr. Frosty freezing container (ThermoFisher, catalog number: 5100-0001 )
- Sterile 35 mm x 10 mm Petri dishes (Corning, catalog number: 430165 )
- Sterile 60 mm x 10 mm Petri dishes (Falcon, catalog number: 351007 )
- Whatman Grade 114 cellulose filter papers (Sigma-Aldrich, catalog number: 1114-185 )
- Warm water (37 °C)
- Liquid nitrogen (-196 °C)
- Dulbecco’s phosphate buffered saline (DPBS)-10x (Sigma-Aldrich, catalog number: 59331C )
- Fibronectin human plasma (Sigma-Aldrich, catalog number: F0895 )
- Human cervical HeLa cancer cell line (ATCC, catalog numbers: CCL-2 )
- Dulbecco's modified Eagle’s medium (DMEM) (Gibco, catalog number: 11965092 )
- Fetal bovine serum (FBS) (Sigma-Aldrich, catalog number: F7524 )
- Penicillin-streptomycin (Pen-Strep) solution (Sigma-Aldrich, catalog number: P4333 )
- Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D2650 )
- TrypLE express enzyme (Gibco, catalog number: 12604021 )
- Corning Matrigel matrix (Sigma-Aldrich, catalog number: DLW356231 )
- Cell counting chamber slides (ThermoFisher, catalog number: C10228 )
- Trypan blue exclusion assay (Optional, Sigma-Aldrich, catalog number: T6146 )
- Invitrogen Live/Dead assay kit for mammalian cells, containing green calcein-AM and red ethidium homodimer-1 fluorescent dyes (ThermoFisher, catalog number: L3224 )
- Complete Roswell Park Memorial Institute (RPMI) medium (Gibco, catalog number: 11875093 )
Equipment
Required
- All-purpose scissors with size of 18 cm
- Ruler of length 15 cm
- High precision tweezers (Dumont, catalog number: 5627 )
- Timer (Sunnex, catalog number: 360594 )
- Microbalance (Mettler Toledo, catalog number: ME54 )
- Autoclave (Runyes, catalog number: SEA 18L-DIG-USB )
- Centrifuge (Eppendorf, catalog number: 5810 )
- Laboratory water bath (Witeg, catalog number: WITEG 20002 )
- Humidifying cell incubator (New Brunswick, catalog number: Galaxy 48R )
- Class II laminar air flow hood (NuAire, catalog number: NU-437S )
- -80 °C freezer (Arctiko, catalog number: ULUF 65 )
- Liquid nitrogen tank (Arpege, catalog number: 40 )
Optional
- Countess II FL automated cell counter (ThermoFisher, catalog number: AMQAF1000 )
- Olympus FV1000 inverted confocal microscope (Olympus America)
Software
- (Optional) Imaris 9.2 image analysis software (Oxford Instruments)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Deliorman, M., Sukumar, P., Alnemari, R. and Qasaimeh, M. A. (2020). A Method to Efficiently Cryopreserve Mammalian Cells on Paper Platforms. Bio-protocol 10(18): e3764. DOI: 10.21769/BioProtoc.3764.
分类
细胞生物学 > 细胞分离和培养 > 低温贮存
细胞生物学 > 细胞分离和培养 > 3D细胞培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link