- EN - English
- CN - 中文
Isolation and ex vivo Expansion of Human Limbal Epithelial Progenitor Cells
人角膜缘上皮祖细胞的分离及体外扩增
发布: 2020年09月20日第10卷第18期 DOI: 10.21769/BioProtoc.3754 浏览次数: 5082
评审: Ralph Thomas BoettcherVishal S ParekhZheng Zachory Wei
Abstract
Limbal stem cell transplantation has been used successfully to treat patients with limbal stem cell deficiency all over the world. However, long term clinical results often proved less satisfactory due to the low quality of the graft or inadequate properties of transplanted cells. To enhance the ex vivo expansion of human limbal epithelial stem or progenitor cells (LEPC) by preserving stem cell phenotype and to improve subsequent transplantation efficiency, cell-matrix interactions ex vivo should mimic the condition in vivo. The laminin isoforms preferentially expressed in the limbal niche can be used as a culture matrix for epithelial tissue engineering. We recently published the expansion of LEPC on various laminin isoforms and observed that laminin alpha 5-derived matrices support the efficient expansion of LEPC compared to tissue culture plates and other laminin isoforms by preserving stem/progenitor cell phenotype. Here, we describe an optimized protocol for the isolation of LEPC from cadaveric corneal limbal tissue by collagenase digestion and efficient expansion of LEPC using recombinant human laminin-511 E8 fragment (LN-511E8) as culture substrate.
Keywords: Limbal epithelial progenitor cells (角膜缘上皮祖细胞)Background
Limbal epithelial stem/progenitor cells (LEPC), responsible for the continuous renewal of the corneal epithelium, reside in a highly specialized and complex environment. It comprises specific extracellular matrix components and supporting limbal niche cells at the corneo-scleral limbus (Schermer et al., 1986; Cotsarelis et al., 1989; Ordonez et al., 2012; Mei et al., 2012). Damage or injury to this stem/progenitor cell reservoir can lead to corneal neovascularization, chronic inflammation, and stromal scarring associated with corneal opacity and loss of vision (Kenyon and Tseng, 1989; Sangwan and Tseng, 2001; Dua et al., 2010). Cultured limbal epithelial transplantation (CLET) has been applied as a therapeutic approach in various clinical centers and the overall success rates of autologous CLET for unilateral cases with a follow-up period of at least 24 months were reported to amount to 72-76% (Rama et al., 2010; Tsai et al., 2010; Utheim et al., 2013; Holland et al., 2015). In the case of bilateral, 34 months of follow-up showed a 70% success rate after allogeneic central penetrating limbo-keratoplasty in conjunction with conjunctivoplasty, mitomycin C, and amniotic membrane transplantation (Eberwein et al., 2012). However, long-term corneal regeneration often proved less satisfactory. This might be caused by the low quality of the graft or inadequate properties of transplanted progenitor cells (Daya et al., 2005; Rama et al., 2010; Pellegrini et al., 2014; Nakamura et al., 2016). These limitations underscore the need for developing novel standardized LEPC culture techniques that ensure the preservation of the stem/progenitor cell phenotype and function during cultivation and after transplantation.
Laminins (LNs) are the best-characterized extracellular matrix constituents present in basement membranes (BM) of adult stem cell niches, including the limbal stem cell niche, where they influence cell behaviors, such as cell adhesion, differentiation, and phenotype stability. Various reports have shown heterogeneity of LN isoform expression in BMs of ocular surface epithelia. Especially LN chains α5, β1, β2, and γ1 are preferentially localized to the BM of the limbal epithelium compared to that of the corneal epithelium (Ljubimov et al., 1995; Schlötzer-Schrehardt et al., 2007; Polisetti et al., 2017) (Figure 1). We have reported earlier that LN alpha 5-containing isoforms as well as the bioactive fragment of human LN-511 (LN-511 E8) support efficient ex vivo expansion of LEPC (Polisetti et al., 2017).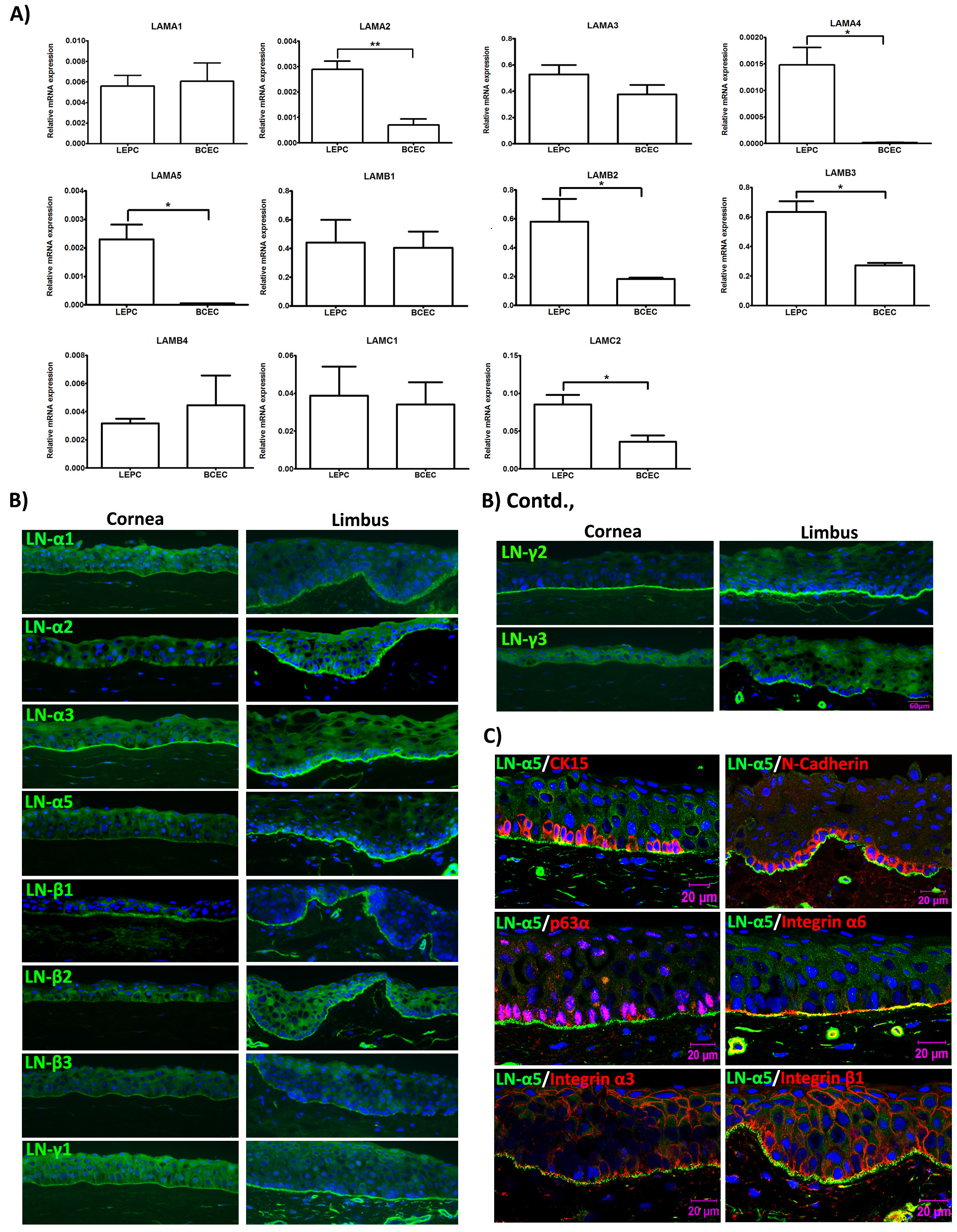
Figure 1. Expression of laminin chains in the limbal stem cell niche in situ. A. Quantitative real-time polymerase chain reaction (qRT-PCR) primer assays showing higher expression levels of laminin α2 (LAMA2), α4 (LAMA4), α5 (LAMA5), β2 (LAMB2), β3 (LAMB3), and γ2 (LAMC2) in microdissected limbal epithelial stem/progenitor cell (LEPC) clusters compared with basal corneal epithelial cell (BCEC) populations; laminin α1 (LAMA1), α3 (LAMA3), β1 (LAMB1), β4 (LAMB4), γ1 (LAMC1) showed no differential expression patterns. B. Immunofluorescence analyses of corneoscleral tissue sections showing differential staining patterns of laminin α2, α5, β2, β3, γ2, and γ3, but similar staining patterns of laminin α1, α3, β1, and γ1 in the basement membranes of corneal and limbal epithelia; laminin α4 was largely negative in epithelial basement membranes. C. Immunofluorescence double labeling of laminin (LN) α5 (green) and cytokeratin (CK)15, N-Cadherin, p63α, integrin α6, integrin α3, and integrin β1 (red); nuclear counterstaining with DAPI (blue); scale bar = 20 µm. Reprinted from Polisetti et al., 2017, licensed under a CC BY 4.0.
Here, we present an optimized protocol for isolation and expansion of LEPC. The isolation protocol is similar to the protocol provided by Chen et al. (2011) with few modifications, but we expanded LEPC on LN-511E8-coated plates to improve proliferation and preservation of a stem/progenitor cell phenotype (Polisetti et al., 2017).
Materials and Reagents
- Micropipette tips (0.5-20 µl, 100-200 µl, 1,000 µl) (Greiner Bio-One)
- 60 mm cell culture dish (Corning, Falcon®, catalog number: 353004 )
- 100 mm cell culture dish (Corning, Falcon®, catalog number: 353003 )
- Syringe filter 0.2 μm (VWR, catalog number: 28145-501 )
- Disposable Scalpel blades No. 10 (pfm Medical ag, Feather®, catalog number: 201000010 )
- Serological pipettes (5 ml, 10 ml) (Corning, StripetteTM)
- 15 ml conical tubes (Greiner Bio-One, catalog number:188271)
- T75 flasks (Corning, catalog number: CLS430641 )
- Reversible cell strainers (Stem Cell Technologies, catalog number:27215)
- Cell filter 20 µm (Sysmex Partec GmbH, Görlitz, Germany)
- Collagenase A (Sigma-Aldrich, Roche Diagnostics, catalog number: 10103578001 )
- Dulbecco’s Phosphate buffered saline (DPBS) (Pan Biotech, catalog number: P04-361000 )
- 0.25% Trypsin-EDTA (Thermo Fisher Scientific, Gibco®, catalog number: 25200056 )
- 0.05% Trypsin-EDTA (Thermo Fisher Scientific, Gibco®, catalog number: 25300054 )
- Dulbecco’s Modified Eagle Medium (DMEM) high glucose (Thermo Fisher Scientific, GibcoTM, catalog number: 11960044 )
- Fetal Bovine Serum (FBS) (Thermo Scientific, GibcoTM, catalog number: 10082147 )
- Penicillin-Streptomycin/Amphotericin B Mixture (Pan Biotech, catalog number: P06-07300 )
- Keratinocyte serum-free medium (KSFM) (Life Technologies, catalog number: 10724-011 )
- Keratinocyte supplements, contains bovine pituitary extract, human recombinant EGF (Life Technologies, catalog number: 37000-015 )
- iMatrix 511(Nippi, catalog number: 892012 )
- 70% Ethanol
- Cytokeratin (CK) 15 (Invitrogen, catalog number: MA5-11344 )
- p63α (Abcam, catalog number: ab32353 )
- CCAAT/enhancer-binding protein delta (Abcam, catalog number: ab139730 )
- CK19 (Abcam, catalog number: ab52625 )
- CK3 (Invitrogen, catalog number: MA1-5763 )
- CK12 (Abcam, catalog number: ab185627 )
- Connexin-43 (Abcam, catalog number: ab235282 )
- Collagenase solution (see Recipes)
- KSFM complete medium (see Recipes)
- Laminin coated plates (see Recipes)
Equipment
- Pipette aid
- Micropipette (P20, P200, P1000)
- Microdissection Scissors
- Forceps
- Scissors
- Hemocytometer
- Biosafety cabinet
- CO2 incubator
- Centrifuge
- Phase contrast inverted microscope with a camera (Objectives 4x, 10x, 20x)
- Freezer -20 °C
- Refrigerator 2-8 °C
Software
- CapturePro 2.10.0.1 (JENOPTIC Optical systems GmbH)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Polisetti, N., Schlunck, G., Reinhard, T., Kruse, F. E. and Schlötzer-Schrehardt, U. (2020). Isolation and ex vivo Expansion of Human Limbal Epithelial Progenitor Cells. Bio-protocol 10(18): e3754. DOI: 10.21769/BioProtoc.3754.
分类
干细胞 > 多能干细胞 > 细胞多能性
细胞生物学 > 细胞分离和培养 > 细胞分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link