- EN - English
- CN - 中文
Immunohistochemistry of Kidney a-SMA, Collagen 1, and Collagen 3, in A Novel Mouse Model of Reno-cardiac Syndrome
心肾综合征新型小鼠模型中肾脏a-SMA,胶原1和胶原3的免疫组化研究
发布: 2020年09月20日第10卷第18期 DOI: 10.21769/BioProtoc.3751 浏览次数: 4396
评审: Xiaoyi ZhengFereshteh AzediAnonymous reviewer(s)

相关实验方案
用于比较人冷冻保存 PBMC 与全血中 JAK/STAT 信号通路的双磷酸化 CyTOF 流程
Ilyssa E. Ramos [...] James M. Cherry
2025年11月20日 2328 阅读
Abstract
Cardiorenal syndrome defines a synergistic pathology of the heart and kidneys where failure of one organ causes failure in the other. The incidence of cardiovascular mortality caused by this syndrome, is 20 fold higher in the end stage renal disease (ESRD) population compared to the population as a whole thus necessitating the need for improved therapeutic strategies to combat reno-cardiac pathologies.
Murine in vivo models play a major role in such research permitting precise genetic modification thus reducing miscellany, however presently there is no steadfast model of reno-cardiac syndrome in the most common genetically modified mouse strain, the C57BL/6 mouse. In this study we have modified an established model of chronic renal disease using adenine diet and extended the associated pathology achieving chronic renal failure and consequent reno-cardiac syndrome in the C57BL/6 mouse.
Eight week-old male C57BL/6 mice were acclimatized for 7 days before administration of a 0.15% adenine diet or control diet for 20 weeks after which the experiment was terminated and blood, urine and organs were collected and analyzed biochemically and by immunohistochemistry.
Administration of 0.15% adenine diet caused progressive renal failure resulting in a reno-cardiac syndrome confirmed by a significantly increased heart to body weight ratio (P < 0.0001). Blood biochemistry showed that adenine fed mice had significantly increased serum creatinine, urea (P < 0.0001), and a significantly reduced glomerular filtration rate (P < 0.05), while immunohistochemistry of the kidneys for α-SMA, collagen 1 and collagen 3 showed severe fibrosis.
We present a novel regimen of adenine diet which induces both chronic kidney disease and reno-cardiac syndrome in the C57BL/6 mouse strain. The non-surgical nature of this model makes it highly reproducible compared to other models currently available.
Background
Chronic kidney disease (CKD) results in vascular and cardiac dysfunction, termed cardio renal syndrome, defined more specifically as reno-cardiac syndrome (RCS) by Ronco et al. (2008). RCS is a predominant feature of end stage renal disease (ESRD) resulting in a 20 fold higher incidence of cardiovascular mortality (compared to the normal population as a whole) and cardiovascular events account for approximately 50% of mortality in ESRD patients (de Jager et al., 2009; Steenkamp et al., 2013). As the ESRD population is increasing worldwide research into novel therapies to combat RCS is vital.
Animal models have significantly aided research into many disease pathologies and the development of technology to genetically modify strains has enabled such models to become even more potent through better targeting of pathological genes.
The sub-total nephrectomy model has been up till now the most successful model of RCS and involves the surgical removal or negation of (5/6ths) of the kidney mass (Morrison, 1962). The resulting renal ischaemia/reduced nephron mass initiates hypertension and subsequent left ventricular hypertrophy (LVH) leading to progressive left ventricular dilatation (Kumar et al., 2014). In parallel, fibroblast activation leads to cardiac hypertrophy and fibrosis (Hewitson, 2012; Bursac, 2014). The disadvantages of this model is that it requires surgical expertise, expensive surgical facilities and that it is prone to inter and intra operator variability. The preferred rodent used in this model is the rat because in mice, this model is much less effective giving highly variable results (Hewitson et al., 2015). Further to this the C57BL/6 mouse strain, the preferred strain for genetic modification (Seong et al., 2004) has been reported to be resistant to the development of CKD by subtotal nephrectomy (Kren and Hostetter, 1999).
An alternative model of CKD is adenine induced. This non-surgical and therefore highly reproducible method was first described by Yokozawa et al. (1982), who noted that the metabolism of adenine differed from that of other purines and that it was nephrotoxic. However this chemical method although very successful in rats, has not flourished in mice because they do not like the adenine taste resulting in rapid weight loss leading to mortality after 4 weeks. Jia et al. (2013), improved the palatability of the diet by the addition of casein, extending the course of the administration to 8 weeks however this is not long enough to achieve RCS.
We found that we could conceal the adenine taste (without the need for addition of casein), by decreasing the concentration of the diet to 0.15% and by beginning its administration when the mice reached 9 weeks of age, extended the course of the regimen to 20 weeks in order to achieve CKD and subsequently RCS as seen by blood biochemistry and heart/bodyweight ratio data (Table 1), immunoblotting and immunohistochemistry.
Therefore we succeeded in developing a highly reproducible model which does not require surgical expertise or facilities (Kieswich et al., 2018).
Although we did not measure blood pressure (BP) we predict that the main cause of the RCS was haemodynamic due to kidney dysfunction and therefore, if BP was measured consciously, e.g., by a telemetric method, this could also prove to be a successful non-surgical model of hypertension.
Table 1. Results showing evidence of RCS. Control group (n = 6) received standard chow for 20 weeks. Adenine treated group (n = 10) received standard chow with the addition of 0.15% adenine for 20 weeks. Compared with animals receiving normal chow, a 20-week diet of 0.15% adenine caused significant increases in plasma urea (P < 0.0001) and creatinine (P < 0.0001), a significantly reduced glomerular filtration rate (GFR) (P < 0.05), and a significantly greater heart weight-to body weight ratio (P < 0.0001).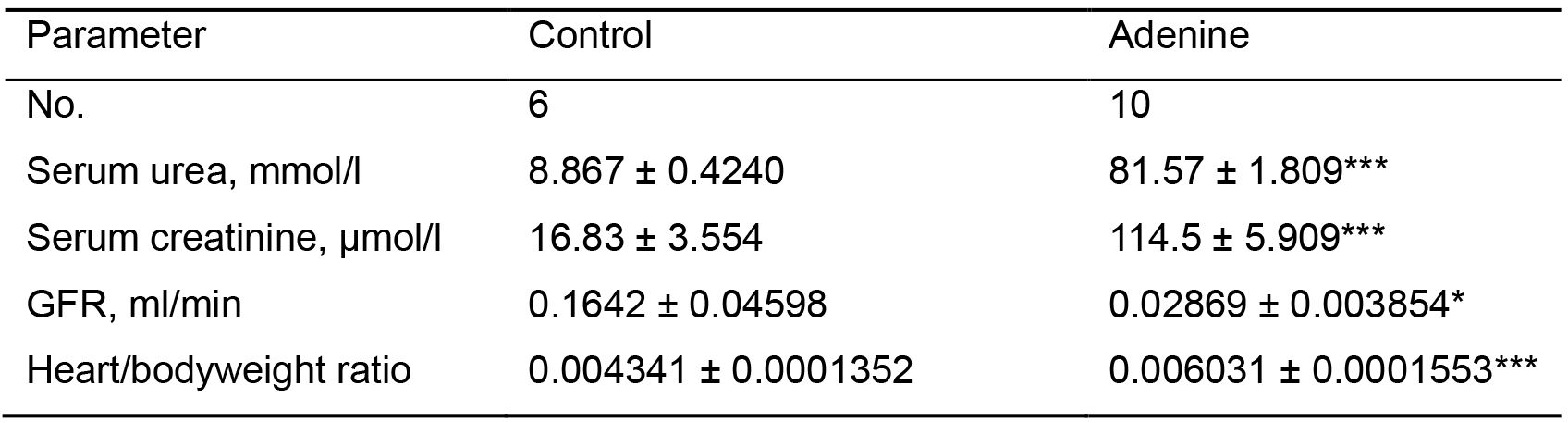
***P < 0.0001,*P < 0.05
Materials and Reagents
- 25 gauge needles (Fisher Scientific, catalog number: 10442204 )
- 1 ml syringes (Fisher Scientific, catalog number: 15849152 )
- Eppendorf tubes (Fisher Scientific, catalog number: 13094697 )
- Embedding cassettes (Fisher Scientific, Simport Acetal Histology Cassettes, catalog number: 10420823 )
- Glass slides (Fisher Scientific, catalog number: 12343138 )
- Pipettes and tips
ErgoOne Single-Channel Pipettes 20-200 µl (STARLAB (UK) Ltd, catalog number: S7100-2200 )
ErgoOne Single-Channel Pipettes 100-1,000 µl (STARLAB (UK) Ltd, catalog number: S7110-1000 )
Yellow Tips (Sterile), Racked (STARLAB (UK) Ltd, catalog number: S1111-0816-C )
1,000 µl Blue Graduated Tip (Sterile), Racked (STARLAB (UK) Ltd, catalog number: S1111-6811-C ) - Hydrophobic barrier pen (Vector Laboratories, ImmEdge Hydrophobic Barrier Pen, catalog number: H-4001 )
- Humidified chamber e.g., a plastic box with airtight lid (prevents tissue from drying out). Place wet tissue paper into the bottom of the box. To keep slides off the wet paper insert a perforated base (can be made by cutting pasteur pipettes into equal size pieces and taping them into a grid formation. Ensure it is level)
- Tissue paper
- Vessel with slide rack to hold approximately 400-500 ml
- Brush (for cleaning microtome blade between samples)
- Glass coverslips (Fisher Scientific, catalog number: 12343138 )
- 8-week old, male C57BL/6 mice (n = 6 per group) (Charles River, Margate, UK)
- 20 Kg Adenine diet (LBS-Biotech, Hookwood, UK, SDS diets, catalog number: 824534 ), RM1 + 0.15% Adenine, storage temperature 4 °C
Note: Buy 20 Kg 824534 RM1 diet (normal adenine concentration) from SDS diets at same time to use as control). For a detailed composition of the diet see attached file ‘Adenine diet composition’. - Ketamine 100 mg/ml solution (Narketan, MWI Animal Health, Somerset, UK, catalog number: 0 3120257 ), storage temperature–Room Temperature (RTP)
- Xylazine hydrochloride 2% w/v solution (Rompun, MWI Animal Health, Somerset, UK, catalog number: 0 2150569 ), storage temperature–RTP
- Buprenorphine 0.3 mg/ml solution (Vertegesic, MWI Animal Health, Somerset, UK, catalog number: 0 1300258 ), storage temperature–RTP
- Heparin Sodium 5,000 u/ml (MWI Animal Health, Somerset, UK, catalog number: 30394030 ), storage temperature–RTP
- Ethanol (Fisher Scientific, catalog number: 10233962 ), storage temperature–RTP
- Phosphate Buffered Saline (Fisher Scientific, PBS, catalog number: 10173433 ), storage temperature 4 °C
- Formalin (Sigma-Aldrich, catalog number: HT5014 ), storage temperature–RTP
- Xylene (Fisher Scientific, catalog number: 10467270 ), storage temperature–RTP
- Parrafin (Fisher Scientific, Thermo Scientific Richard-Allan Scientific Histoplast Paraffin, catalog number: 12683026 ), storage temperature–RTP
- Hydrogen peroxide (Fisher Scientific, H2O2, catalog number: 10687022 ), storage temperature 4 °C
- Triton X-100 (Sigma-Aldrich, catalog number: T8787 ), storage temperature–RTP
- Bovine Serum Albumin (Fisher Scientific, BSA, catalog number: 11423164 ), storage temperature 4 °C
- Primary antibody, anti α-Smooth muscle actin antibody (Abcam, catalog number: ab5694 ), storage temperature -20 °C
- Primary antibody, anti Collagen I antibody (Abcam, catalog number: ab21286 ), storage temperature 4 °C
- Primary antibody, anti Collagen III antibody (Abcam, catalog number: ab7778 ), storage temperature 4 °C
- Secondary antibody, Goat Anti-Rabbit IgG H&L (HRP) (Abcam, catalog number: ab205718 ), storage temperature 4 °C
- Normal Goat serum (Abcam, catalog number: ab7481 ), storage temperature 4 °C
- DAB (Fisher Scientific, catalog number: 10006913 ), DAB storage temperature -20 °C. DAB Substrate kit storage temperature 4 °C
- Glacial acetic acid (Sigma-Aldrich, catalog number: A6283 ), storage temperature–RTP
- TRIS hydrochloride (Tris-HCl) (Sigma-Aldrich, catalog number: RES3098T-B7 ), storage temperature RTP
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888 ), storage temperature–RTP
- Ammonium hydroxide (NH4OH) 30% solution (Sigma-Aldrich, catalog number: 221228-1L-A ), storage temperature RTP
- Sodium citrate (Sigma-Aldrich, Tri-sodium citrate (dihydrate), catalog number: W302600 ), storage temperature RTP
- Tween 20 (Sigma-Aldrich, catalog number: 93773 ), storage temperature RTP
- Hematoxylin (Vector Laboratories Ltd, VECTOR®HEMATOXYLINNUCLEAR COUNTERSTAIN, catalog number: H-3401 ), storage temperature RTP
- Mounting medium (Vector Laboratories, VectaMount Permanent Mounting Medium, catalog number: H-5000 ), storage temperature RTP
- Hydrochloric acid (Sigma-Aldrich, catalog number: 258148 ), storage temperature RTP
- Bluing solution (see Recipes)
- Acid rinse solution (see Recipes)
- Sodium citrate buffer (see Recipes)
- TBS buffer (see Recipes)
Equipment
- Dissection Forceps (Harvard Apparatus, Jewelers Forceps, No. 7, catalog number: 72-8685 )
- Scissors (Harvard Apparatus, Eye Scissors, Straight, Sharp/Sharp, catalog number: 2-8438 )
- Ear punch for identifying mice (Harvard Apparatus, Model 4.7.2 Forceps Type Punch, 2.0 mm hole, catalog number: 34-0137 )
- Fume hood (Fisher Scientific, Thermo Scientific Hyperclean TruAir Ductless Fume Hood, catalog number: 15613136 )
- Orbital shaker (Fisher Scientific, Stuart Orbital Shaker, catalog number: 10759145 )
- Vacuum Oven (Fisher Scientific, Technico, catalog number: 13025703 )
- Microtome and blade, Fisher Scientific, HM 325 Rotary Microtome, catalog number: 12052999 )
- Waterbath (Fisher Scientific, Clifton Unstirred Digital Water Bath, catalog number: 15700619 )
- Domestic stainless steel pressure cooker (e.g., Argos, Tower Compact 4 Litre Pressure Cooker, catalog number: 711/5008 )
- Hot plate (Fisher Scientific, Stuart Hotplate with Stirrer, catalog number: 11966558 )
- Centrifuge (Fisher Scientific, Thermo Scientific Heraeus Megafuge 8, catalog number: 15211026 )
- Microscope (3D Histech Ltd, Budapest, Hungary, Panoramic Scanning 250 microscope)
- Refrigerator (Fisher Scientific, Loughborough, UK, Liebherr, 12088281)
- Staining set consisting of multiple containers (Tissue Tek Slide Staining Dish, Sakura Finetek Europe, Alphen aan den Rijn, The Netherlands, S76316)
Software
- Panoramic Viewer software (3D Histech Ltd, Budapest, Hungary)
- ImageJ software (https://imagej.nih.gov)
- Excel (https://products.office.com/en-gb/excel)
- GraphPad Prism (https://www.graphpad.com/scientific-software/prism/)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Kieswich, J. E., Chen, J., Alliouachene, S., Caton, P. W., McCafferty, K., Thiemermann, C. and Yaqoob, M. M. (2020). Immunohistochemistry of Kidney a-SMA, Collagen 1, and Collagen 3, in A Novel Mouse Model of Reno-cardiac Syndrome. Bio-protocol 10(18): e3751. DOI: 10.21769/BioProtoc.3751.
分类
免疫学 > 动物模型 > 小鼠
分子生物学 > 蛋白质 > 磷酸化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link