- EN - English
- CN - 中文
Ratiometric Measurement of Protein Abundance after Transient Expression of a Transgene in Nicotiana benthamiana
转基因烟草瞬时表达后蛋白质丰度的比例计量测定
发布: 2020年09月05日第10卷第17期 DOI: 10.21769/BioProtoc.3747 浏览次数: 5976
评审: Shin‐nosuke HashidaGazala AmeenAnonymous reviewer(s)

相关实验方案

通过简并PCR鉴定二倍体马铃薯Solanum okadae中的S位点F-box蛋白序列
Amar Hundare [...] Timothy P. Robbins
2025年06月05日 1991 阅读
Abstract
Ratiometric reporters are tools to dynamically measure the relative abundance of a protein of interest. In these systems, a target protein fused to a fluorescent or bioluminescent reporter is expressed with fixed stoichiometry to a reference protein fused to a second reporter. Both fusion proteins are encoded on a single transcript but are separated during translation by a 2A “self-cleaving” peptide. This approach enables changes in the relative abundance of a target protein to be detected sensitively, reducing variability in expression of the ratiometric reporter transgene that may occur across different tissues or transformation events. We recently developed a set of Gateway-compatible plant transformation vectors termed pRATIO that combine a variety of promoters, fluorescent and bioluminescent reporters, and 2A peptides derived from foot-and-mouth disease virus. Here, we describe in detail how to use the dual-fluorescent ratiometric reporter pRATIO3212 to examine the relative abundance of a target protein after transient expression in Nicotiana benthamiana leaves. For this example, we analyze degradation of the SUPPRESSOR OF MAX2 1 (SMAX1) protein from Arabidopsis thaliana in response to treatments with karrikins and rac-GR24. This protocol provides a simple, rapid, and readily scalable method for in vivo analysis of relative protein abundance in Agrobacterium-infiltrated Nicotiana leaf tissues.
Keywords: Ratiometric reporter (比例报告器)Background
Karrikins (KARs) are butenolide compounds found in smoke that stimulate seed germination and enhance seedling photomorphogenesis of Arabidopsis thaliana (Flematti, 2004; Nelson et al., 2009, 2010 and 2012). KAR responses in Arabidopsis require the α/β-hydrolase KARRIKIN INSENSITIVE2/HYPOSENSITIVE TO LIGHT (KAI2/HTL) (Sun and Ni, 2011; Waters et al., 2012). KAI2 can bind KAR1 in vitro and is thought to function as a KAR receptor (Guo et al., 2013). KAI2 works with the F-box protein MORE AXILLARY GROWTH2 (MAX2) to mediate KAR responses, likely through polyubiquitination and degradation of SUPPRESSOR OF MAX2 1 (SMAX1) (Nelson et al., 2011; Stanga et al., 2013; Waters et al., 2017). This signaling mechanism is highly similar to that of the plant hormone strigolactone (SL). In SL signaling, DWARF14 (D14)/DECREASED APICAL DOMINANCE2 (DAD2), which is an ancient paralog of KAI2, works with MAX2 to target a subset of SMAX1-LIKE proteins for degradation (Hamiaux et al., 2012; Waters et al., 2012; Jiang et al., 2013; Zhou et al., 2013; Soundappan et al., 2015; Wang et al., 2015). Interestingly, KAI2 and D14 are both responsive to rac-GR24, a commonly used racemate of synthetic SL analogs. However, they show different preferences for the two enantiomers in this mixture, only one of which has a stereochemical configuration that mimics natural SLs (Scaffidi et al., 2014; Waters et al., 2015; Flematti et al., 2016).
In addition to mediating KAR responses, there is growing evidence that the primary role of KAI2 is the recognition of an unknown endogenous signal known as KAI2 ligand (KL) (Conn and Nelson, 2015). Identification of KL will require a highly specific assay for the activation of KAI2. Recently, we found evidence that SMAX1 degradation occurs after KAR and rac-GR24 treatment, and this response requires MAX2 and KAI2 (Khosla et al., 2020b). This observation led us to develop a bioassay for SMAX1 degradation that is appealing as a direct and specific readout of KAI2 activation.
We generated a series of ratiometric reporter vectors (pRATIO) that can be used to assess protein abundance in vivo by monitoring the relative abundance of two co-expressed fluorescent or bioluminescent reporters, one of which is fused to a target protein of interest (Khosla et al., 2020a). Rather than express a pair of target and reference genes from two promoters on a single vector or two co-transformed vectors, in the pRATIO system the target and reference genes are transcribed on the same mRNA. Importantly, pRATIO vectors can normalize for differences in transformation efficiency or transgene expression across samples by encoding a 2A ribosomal skipping peptide from foot-and-mouth disease virus (FMDV) between the target and reference genes. During translation, the nascent 2A peptide blocks the ribosomal exit channel. This disrupts formation of the bond between the C-terminal Gly and Pro residues of the 2A peptide. Translation can resume on the mRNA, producing two proteins in near stoichiometric ratios (Luke and Ryan, 2018).
Here we present a detailed protocol for using the pRATIO system. As an example, we monitor KAR- and rac-GR24- induced proteolysis of Arabidopsis SMAX1 that has been transiently expressed in Nicotiana benthamiana.
Materials and Reagents
- CorningTM 96-Well Black Polystyrene Microplates (Fisher, catalog number: 07-200-590 )
- 5 ml Centrifuge tubes (VWR, catalog number: 10002-731 )
- Polystyrene culture tubes (VWR, catalog number: 60818-703 )
- 1 ml Norm-Ject Syringes (Fisher, catalog number: 1481725 )
- 50 ml Falcon tubes (Genesee Scientific, catalog number: 28-108 )
- 24-Well, Flat Bottom plates (Olympus, catalog number: 25-102 )
- 96-Well Microplates, Clear (Greiner Bio-One, catalog number: 655801 )
- Potting soil (Professional Growing Mix, sungro Horticulture) supplemented with Gnatrol WDG, Marathon (imidacloprid), and Osmocote 14-14-14 fertilizer
- Wild type Nicotiana benthamiana seeds (seeds kindly provided by Dr. Martha Orozco-Cardenas; Plant Transformation Research Center)
- Agrobacterium tumefaciens strain GV3101 strain with helper plasmid pMP90 (Koncz and Schell, 1986; kindly provided by Dr. Meng Chen, University of California, Riverside)
- pRATIO3212-SMAX1 plasmid (Khosla et al., 2020b)
- Agrobacterium tumefaciens strain GV3101 with helper plasmid pMP90 (Koncz and Schell, 1986) carrying pBIN61-p19 construct (Habibi et al., 2018)
- Antibiotics
Gentamicin Sulfate (GoldBio, catalog number: G-400-10 )
Spectinomycin Dihydrochloride Pentahydrate (GoldBio, catalog number: S-140-5 )
Kanamycin Monosulfate (GoldBio, catalog number: K-120-10 )
Rifampicin (GoldBio, catalog number: R-120-1 ) - Magnesium chloride hexahydrate (MgCl2·6H2O) (Fisher, catalog number: 14222322 )
- 2-(N-Morpholino) ethanesulfonic acid (MES) (Fisher, catalog number: BP300100 )
- Potassium hydroxide (KOH) (Fisher, catalog number: P250-500 )
- Acetosyringone (Fisher, catalog number: AC115540010 )
- Dimethyl Sulfoxide (DMSO) (Fisher, catalog number: BP231-100 )
- Luria-Bertani (LB) broth (Fisher, catalog number: BP9723-2 )
- Bacteriological agar (Sigma, catalog number: A5306-1KG )
- Solid LB plates with 1.5% (w/v) agar
- KAR1
- KAR2
- rac-GR24
- Acetone
- MgCl2 (1 M stock) (see Recipes)
- MES (0.5 M stock) (see Recipes)
- Acetosyringone (1 M stock) (see Recipes)
- Infiltration Buffer (see Recipes)
- KAR1, KAR2, rac-GR24 (50 mM stock) (see Recipes)
Notes:- KAR2 was synthesized as previously reported (Goddard-Borger et al., 2007). rac-GR24 was synthesized according to Mangus et al. and recrystallized from diethyl ether/hexanes (Mangnus et al., 1992).
- The chemicals can be purchased from several commercial suppliers: KAR1: Toronto Research Chemicals (M305480), Chiralix (CX27716); KAR2: Toronto Research Chemicals (F864800), Chiralix (CX94877); rac-GR24: Chiralix (CX23880), Strigolab (ST23b rac), PhytoTech Labs (G3324), Toronto Research Chemicals (S687590).
- 10 µM working chemical stocks (see Recipes)
- 0.02% (v/v) acetone control (see Recipes)
Equipment
- Centrifuge (Eppendorf, model: 5804 R )
- 30 °C incubator shaker (ThermoFisher, model: MaxQ 6000 R )
- CLARIOstar microplate reader (BMG Labtech)
- 4 mm Round Ticket Hole Punch
- Dumont #5 Fine Forceps (Fine Science Tools, catalog number: 11252-20 )
- 1 mm electroporation cuvette (Genesee Scientific, catalog number: 40-100 )
- MicroPulser Electroporator (Bio-Rad, catalog number: 165-2100 )
- Vacuum-driven filter system (Olympus, catalog number: 25-227 )
- 3.5-inch square plastic pots (Farrand, catalog number: K0-25SQ-G )
Software
- Prism (v8.2.0, GraphPad Software Inc.)
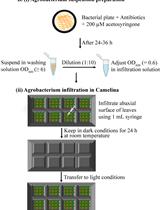
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Khosla, A. and Nelson, D. C. (2020). Ratiometric Measurement of Protein Abundance after Transient Expression of a Transgene in Nicotiana benthamiana. Bio-protocol 10(17): e3747. DOI: 10.21769/BioProtoc.3747.
分类
植物科学 > 植物分子生物学 > 蛋白质
分子生物学 > 蛋白质 > 靶向降解
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link