- EN - English
- CN - 中文
In vivo Blood-brain Barrier Permeability Assays Using Clostridium perfringens Epsilon Toxin
应用产气荚膜梭菌ε毒素测定体内血脑屏障通透性
发布: 2020年08月05日第10卷第15期 DOI: 10.21769/BioProtoc.3709 浏览次数: 6757
评审: Alexandros AlexandratosSubhi MarwariHSIU CHUN CHUANG
Abstract
In order for the brain to function properly, a carefully orchestrated homeostasis must be maintained. To help regulate this delicate balance, the brain has developed a highly selective blood-brain barrier (BBB). Under normal conditions, the BBB excludes harmful blood-borne material from the brain parenchyma. However, numerous neuropathological conditions can disrupt this barrier, causing BBB permeability and subsequent CNS dysfunction. Understanding the mechanisms involved in BBB permeability are essential to elucidating the pathology of various neurological disorders as well as identifying methods for drug delivery to the CNS. Here, we describe several in vivo methods to measure BBB permeability in mice using an array of diverse sized tracers including exogenous 376 Da fluorescein salt, 66.5 kDa bovine serum albumin, and 70 kDa dextran as well as endogenous 160 kDa mouse IgG. When administered intravenously, these substances are excluded from a healthy brain by the BBB. However, BBB dysfunction can allow entry of these tracers into the brain and this accumulation can be measured using spectrophotometry, fluorescent microscopy, and immunohistochemistry. We also describe a method to induce BBB permeability using Clostridium perfringens epsilon toxin. Finally, we include a short discussion about the advantages and disadvantages of each method and their appropriate downstream applications.
Background
To maintain the intricate homeostasis required for proper brain function, the central nervous system (CNS) has developed a selective barrier that stringently regulates movement of blood-borne material into the brain. This is called the blood-brain barrier (BBB). This barrier is generated by highly specialized brain endothelial cells (BEC) whose unique properties are influenced by the close contact of CNS pericytes and astrocytes (Balabanov and Dore-Duffy, 1998; Engelhardt, 2003; Abbott et al., 2006; Abbott and Friedman, 2012; Alvarez et al., 2013). This increased barrier activity is achieved using two main mechanisms in BEC. The first is reduced paracellular permeability via formation of tight junctions at cell-to-cell contacts and the second is decreased transcellular permeability through reduced pinocytic activity (Gloor et al., 2001; Wolburg and Lippoldt, 2002; Preston et al., 2014; Tietz and Engelhardt 2015; De Bock et al., 2016). An increase in BBB permeability can have devastating neuropathological outcomes and can result in death. Because of the destructive consequences that BBB permeability can have on normal brain function, it is important to establish methods that reliably measure BBB permeability to help identify both the causes of and treatments for BBB dysfunction.
To measure BBB permeability in vivo in mice, animals are typically injected intravenously (IV) with a tracer or dye normally excluded from the brain by an intact BBB. The most commonly used tracer is Evans Blue (Saunders et al., 2015). However, it has been argued that Evans Blue has significant limitations as a tracer for BBB permeability assays and has been extensively reviewed in Saunders et al. (2015). Because Evan Blue binds to plasma albumin, extraversion of Evans Blue into the CNS is reasoned to be a measurement of albumin permeability. However, it has been demonstrated that Evans Blue binds to several other plasma proteins and may even be present as a free dye, suggesting that Evans Blue is an unsuitable tracer for BBB permeability. To help reduce the amount of uncertainty of accessing BBB permeability with Evan’s Blue, we sought to identify tracers that were better characterized and would therefore allow us to better define the specific aspects involved in BBB dysfunction. For our purposes, we wished to identify the size of molecules a damaged BBB had become permeable to, indicating if certain blood proteins could cross the BBB. Extravasation of certain blood-borne proteins such as complement, fibrinogen, and immunoglobulins may have lasting pathological effects on the CNS even after a permeable BBB has been repaired. Based on these criteria, we selected a panel of easily detectable tracers including exogenous 376Da fluorescein salt (FITC-Na), 66.5 kDa Alexa fluor 594 conjugated bovine serum albumin (BSA-594), and 70 kDa fluorescein isothiocyanate conjugated dextran (FITC-dextran); and the endogenous ~155 kDa tracer, mouse IgG.
In this paper, we outline procedures on how to use FITC-Na, BSA-594, -FITC-dextran, and endogenous IgG as tracers for BBB permeability and their acceptable downstream applications. In addition, we also describe a method to induce BBB permeability in mice using Clostridium perfringens epsilon toxin (ETX) (Linden et al., 2019). ETX-induced BBB permeability opens the BBB to all the tracers described here, specifically through caveolae dependent-transcytosis using both receptor-mediated and fluid-phase mechanisms (Linden et al., 2019). This panel allows us to access permeability to substances with varied molecular sizes and well as different molecular characteristics. FITC-Na can be used as a measure of solute and ion permeability, while BSA, 70 kDa dextran, and IgG can be used as measures of protein permeability (Nag, 2003). Influx of specific markers may also help identify what paracellular or transcellular mechanisms are involved in BBB dysfunction. For example, albumin transport is mediated via caveolae dependent-transcytosis (Schnitzer et al., 1994; Schubert et al., 2001; Frank et al., 2003). However, specific mechanisms of BBB permeability need to be confirmed using additional experimental methods. We strongly suggest ultrastructure examination via electron microscopy to determine if permeability is a result of increased paracellular permeability via tight junction dysfunction or increased transcellular permeability because of elevated endocytic activity. We also describe the best methods to use to evaluate extravasation for each tracer, as some techniques are not compatible with certain tracers, and can result in significant reduction of signal (Buxton, 1978; Hoffmann et al., 2011, Saunders et al., 2015). Finally, we also describe some of the advantages and disadvantages for each of the methods and tracer combinations (Figure 1). These methods may be used to measure BBB permeability in numerous disease models, transgenic mouse models, and CNS drug delivery evaluations.
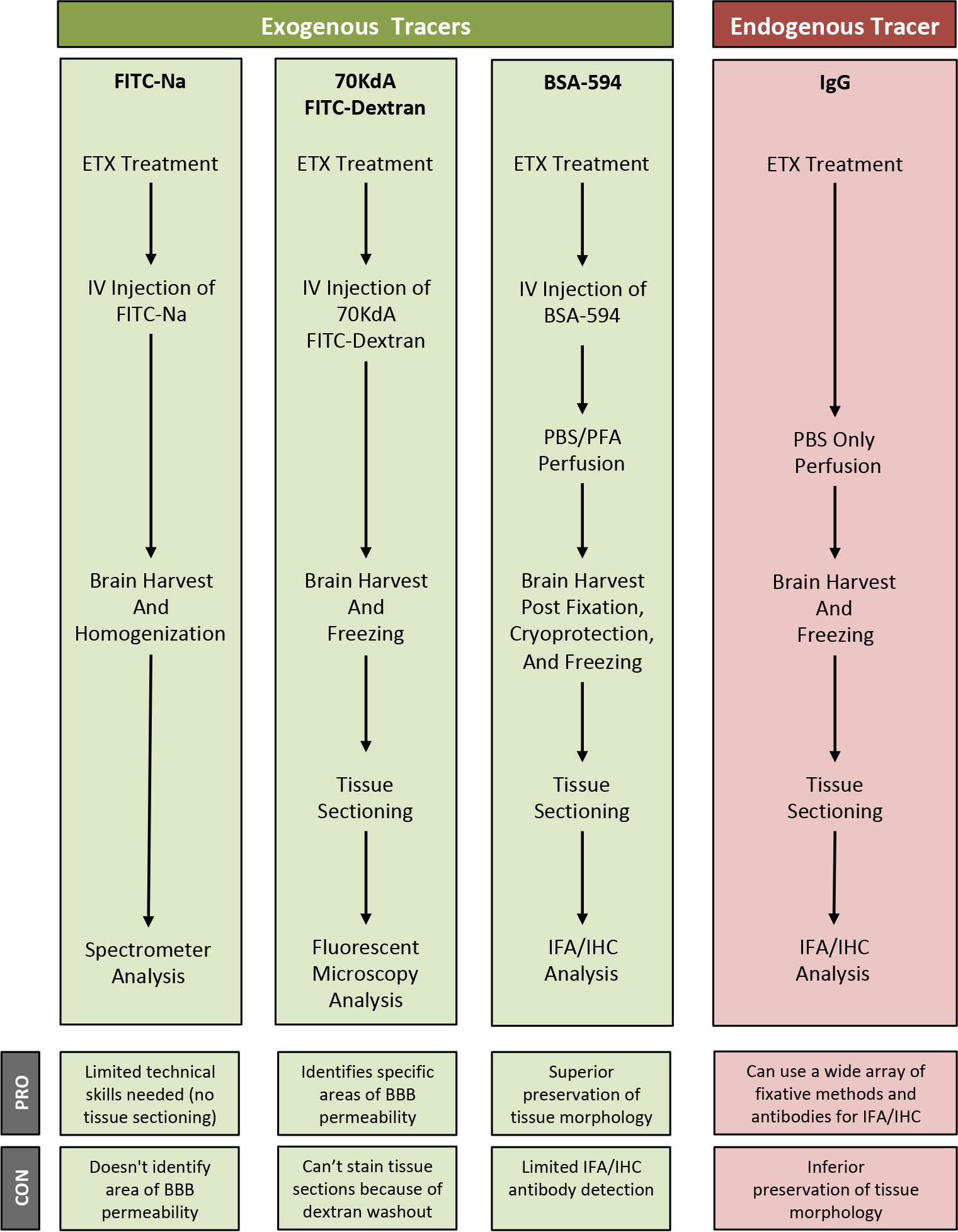
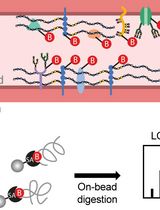
Figure 1. Overview of specific tracer and post-treatment workflow highlighting advantages (PRO) and disadvantages (CON) of each method
Materials and Reagents
- Clear nail polish (any brand)
- Microscope slide storage box (any brand)
- Razor blades (any brand)
- 2 mm glass beads (Sigma, catalog number: Z273627 )
- Microcentrifuge tube (Sigma, catalog number: CLS320 )
- Kimwipes
- Insulin Syringes, 29 Gauge, 0.5 ml volume, 12.7 mm needle length (BD, catalog number: 324703 )
- Intermediate Size Cryomolds (Tissue-Tek, catalog number: AGG4582 )
- 22 x 50 mm Rectangular #1½ Cover Glass (Corning, catalog number: 2980-225 )
- Superfrost Plus Microscope Slides (Fisherbrand, catalog number: 12-550-15 )
- C57BL/6J mice, female, 8 weeks old (The Jackson Laboratory, catalog number: 000 664 )
- 70% Ethanol
- Epsilon protoxin (Biodefense and Emerging Infections Research Resources Repository, catalog number: NR-856 ), store at -80 °C
- Immobilized TPCK-Trypsin resin (Thermo Scientific, catalog number: 20230 ), store at 4 °C
- Sodium phosphate monobasic dehydrate (Sigma, catalog number: 71505 )
- Sterile Saline Solution: 0.9% sodium chloride solution, 10 ml vial, Single-dose, for use as sterile diluent (Hospia, Inc.), store at room temperature
- FITC-Na (Fluorescein sodium salt) (Sigma, catalog number: F6377 ), store at room temperature protected from light
- 70 kDa FITC-dextran (Fluorescein isothiocyanate-dextran with average molecular weight of 70,000 Da) (Sigma, catalog number: 46945 ), store at 4 °C protected from light
- BSA-594 (Albumin from Bovine Serum), Alexa Fluor 594 conjugated (Thermo Fisher Scientific, catalog number: A13101 ), store at 4 °C protected from light
- PBS (Invitrogen, catalog number: AM9625 ), store at room temperature
- Xylazine and ketamine cocktail, obtained from institutional Research and Animal Resource Center or equivalent
- Tissue-Tek® OCT Compound (Sakura Finetek, catalog number: 4583 ), store at room temperature
- Pellet and crushed/powdered dry ice
- 4% Paraformaldehyde (PFA) in PBS (Boston BioProducts, catalog number: BM-155 ), store at 4 °C for short term storage or -20 °C for long term storage
- Triton X-100 (Sigma, catalog number: T-8787 )
- Rabbit anti-PDGFR beta antibody (Abcam, catalog number: ab32570 )
- FITC-BSL1: Fluorescein labeled Griffonia (Bandeiraea) Simplicifolia Lectin I (GSL I, BSL I), (Vector labraotries, catalog number: F-1101 ), store at 4 °C protected from light
- Antifade Mounting Medium with DAPI (Vectasheild, catalog number: H-1200 ), store at 4 °C protected from light
- Trizma Base (Sigma, catalog number: T1503 )
- Proteinase K (QIAGEN, catalog number: 19131 ), store at room temperature
- EDTA (Sigma, catalog number: EDS )
- Glycerol (Sigma, catalog number: G5516 )
- Fetal bovine serum (FBS) (Sigma, catalog number: F4135 )
- Trypsin digestion buffer (see Recipes)
- 6 mg/ml FITC-Na Solution (see Recipes)
- 20 mg/ml 70 kDa FITC-dextran Solution (see Recipes)
- 1.5 mg/ml BSA-594 (see Recipes)
- 1x PBS (see Recipes)
- 30% sucrose (any brand) solution in PBS, store at 4 °C for up to four weeks (see Recipes)
- Antibody Diluent (see Recipes)
- Cy3 conjugated AffiniPure Goat Anti-Mouse IgG (H+L) (Jackson ImmunoResearch Laboratories, catalog number: 115-165-003 ), store at -20 °C (see Recipes)
- FITC conjugated AffiniPure Donkey Anti-Rabbit IgG (H+L) (Jackson ImmunoResearch Laboratories, catalog number: 711-095-152 ), store at -20 °C (see Recipes)
- Proteinase K antigen retrieval solution (see Recipes)
Equipment
- -80 °C freezer
- Tabletop microcentrifuge (Beckman Coulter, model: Microfuge 18 centrifuge , catalog number: 367160 )
- Tube Shaker and Rotator (Thermo Scientific, model: 4002110Q )
- Surgical equipment for transcardial perfusion and brain harvest (Gage et al., 2012; Sultan, 2013)
- Analytical balance that can measure micrograms (Sartorius, catalog number: CPA2202S )
- Tabletop vortexer (Scientific Industries, catalog number: (G560)SI-0236)
- Centrifuge that can accommodate 15 ml conical tubes (Eppendorf, model: 5810 R , catalog number: 022625101 )
- Spectrophotometer (Eppendorf, model: BioPhotometer Plus , catalog number: 6132 )
- Cuvets (UVette Cuvets) (Eppendorf, catalog number: 952010069 )
- Transcardial perfusion apparatus (Gage et al., 2012)
- Styrofoam cooler or equivalent containers to hold dry ice
- Stainless steel tray or equivalent that can fit into container holding dry ice
- Slide warmer (Marshall Scientific, model: 26020 )
- Cryostat (Leica, model: CM3050 S )
- Fluorescent microscope with green (FITC), red (Cy3), and blue (DAPI) channels and camera (Zeiss Axioskop2 Plus upright microscope and Spot RT3 camera with Spot RT3 camera and Spot Imaging software)
Software
- Spot Imaging software
- Adobe Photoshop
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Mazzucco, M. R., Vartanian, T. and Linden, J. R. (2020). In vivo Blood-brain Barrier Permeability Assays Using Clostridium perfringens Epsilon Toxin. Bio-protocol 10(15): e3709. DOI: 10.21769/BioProtoc.3709.
分类
神经科学 > 神经系统疾病 > 血脑屏障
细胞生物学 > 组织分析 > 生理学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link