- EN - English
- CN - 中文
A Quantitative Single-cell Flow Cytometry Assay for Retrograde Membrane Trafficking Using Engineered Cholera Toxin
使用基因工程霍乱毒素进行逆行膜转运的定量单细胞流式细胞术分析
发布: 2020年08月05日第10卷第15期 DOI: 10.21769/BioProtoc.3707 浏览次数: 5049
评审: Alessandro DidonnaDana Manuela SavulescuAnonymous reviewer(s)

相关实验方案
外周血中细胞外囊泡的分离与分析方法:红细胞、内皮细胞及血小板来源的细胞外囊泡
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
2025年11月05日 1328 阅读
Abstract
The organization and distribution of proteins, lipids, and nucleic acids in eukaryotic cells is an essential process for cell function. Retrograde trafficking from the plasma membrane to the Golgi and endoplasmic reticulum can greatly modify cell membrane composition and intracellular protein dynamics, and thus typifies a key sorting step. However, methods to efficiently quantify the extent or kinetics of these events are currently limited. Here, we describe a novel quantitative and effectively real-time single-cell flow cytometry assay to directly measure retrograde membrane transport. The assay takes advantage of the well-known retrograde trafficking of cholera toxin engineered with split-fluorescent proteins to generate novel tools for immediate monitoring of intracellular trafficking. This approach will greatly extend the ability to study the underlying biology of intracellular membrane trafficking, and how trafficking systems can adapt to the physiologic needs of different cell types and cell states.
Keywords: Retrograde membrane transport (逆行膜运输)Background
All eukaryotic cells depend on their ability to dynamically sort and sequester molecules to membrane-bound subcellular organelles for organization and distribution to specific regions of the cell. An essential step in this process is the early sorting endosome and trans-Golgi network (TGN). Among other trafficking pathways that emerge from the sorting endosome, retrograde trafficking via the secretory pathway to the TGN typifies a key sorting step (Johannes and Popoff, 2008). Cell membrane proteins and lipids undergo retrograde trafficking. Further retrograde trafficking from the TGN all the way to the endoplasmic reticulum (ER) can be completed by some plasma membrane lipids and can cleverly be co-opted by several bacterial toxins and viruses to cause disease (Cho et al., 2012; Personnic et al., 2016; Williams and Tsai, 2016).
Previous biochemical assays measured sulfation and N-glycosylation of toxin as an indication of entry into the TGN and ER, respectively (Rapak et al., 1997; Falguieres et al., 2001; De Luca and Lencer, 2006). These biochemical assays, and alternatively the use of ultrastructural electron and confocal light microscopy, have proven to be highly informative (Sandvig et al., 1992; Torgersen et al., 2001; Chinnapen et al., 2012). However, these approaches can be laborious, have poor dynamic range and are difficult to quantify with precision.
It is well established that AB5 bacterial toxins undergo endocytosis and retrograde transport to enter the TGN and ER (Lencer and Tsai, 2003). Cholera toxin (CTx) which typifies the AB5 toxin family, consists of an individual catalytic A subunit (CTA) that includes the CTA1 and CTA2 subregions. CTA is non-covalently associated with the homopentameric B subunits (CTB) through CTA2 subregion. CTB binds the glycosphingolipid GM1 receptor at the plasma membrane and initiates endocytosis. Following endocytosis, CTx enters the ER via retrograde transport. Here, the enzymatically active CTA1-chain dissociates and undergoes retro-translocation to escape the ER and enter the cytosol. Once in the cytosol, the CTA1-chain refolds and activates adenyl cyclase to cause disease. Meanwhile, the CTA2-chain remains assembled with the B-subunit in the ER lumen.
We took advantage of CTx’s structural features that underlie retrograde trafficking to develop a novel quantitative, sensitive and near real-time single-cell flow cytometry assay. The assay depends on CTB’s ability to bind GM1 at the cell membrane and the C-terminal KDEL-motif of the CTA2-chain’s ability to bind the ER-retention KDEL-receptor (Lencer and Tsai, 2003). Additionally, we applied the split fluorescent protein (spilt-FP) technologies to CTx for single-cell flow cytometry (Feng et al., 2017; Luong et al., 2020). The CTA2 chain was engineered to include the split monomeric neon green2 (mNG211) peptide and was coexpressed in E. coli with the homopentameric CTB. The assembled CTA2-mNG211 chain associated with the CTB pentamer was purified, hereon called CTB-mNG211 (Figure 1A).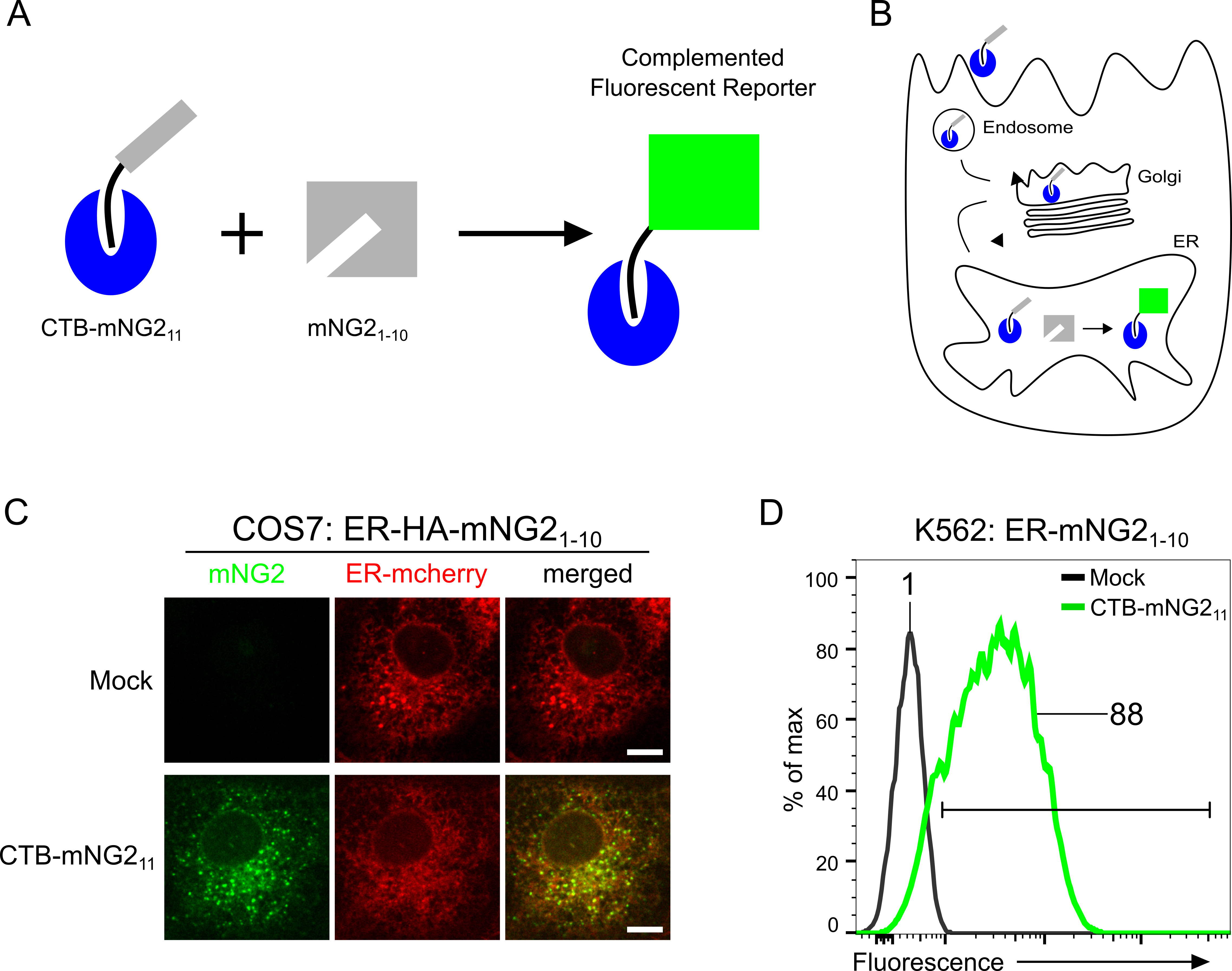
Figure 1. Split-fluorescent toxin reporter for retrograde trafficking. A. Cartoon schematic of split-fluorescent toxin reporter strategy. The CTA2-mNG211 strand (black-gray) is assembled non-covalently to cholera toxin B subunit (CTB, blue) to recombinantly produce the CTB-mNG211 toxin reporter. Complementation of CTB-mNG211 with mNG21-10 produces green fluorescence. B. Retrograde trafficking of CTB-mNG211 and complementation of ER-localized mNG21-10. ER-localized complementation generates a green fluorescent signal. C. Confocal microscopy of CTB-mNG211 retrograde trafficking to the ER in COS7 cells stably expressing ER-mcherry and HA-tagged ER-HA-mNG21-10. Cells were incubated with CTB-mNG211 for 6 h. Scale bar = 10 μm. C. Flow cytometry graph of relative fluorescence intensities of retrograde trafficking from the plasma membrane to the ER. K562 cells stably expressing K562-ER-HA-mNG21-10 were Mock or CTB-mNG211 treated for 5 h and assayed by flow cytometry.
The assay also requires cell-lines of interest to stably express the complementary portion of the split neon green2 fragment (mNG21-10) into organelle compartments of the retrograde pathway. We recently engineered reporter systems to monitor retrograde trafficking from the plasma membrane into the Trans-Golgi Network (TGN) and also the Endoplasmic Reticulum (ER) (Luong et al., 2020). Various cell lines including adherent epithelial cells (e.g., human embryonic kidney (HEK 293T) and African Green Monkey kidney COS7) and cells grown in suspension (e.g., human lymphocyte K562 cells) have been validated for retrograde TGN and ER retrograde trafficking (Luong et al., 2020). Some cell lines may not have GM1 lipid and thus will normally not be suitable for the toxin reporter assay. Insufficient GM1 expression can be adapted for the assay by pre-incubating cells with exogenous GM1 containing short C12:0 acyl chain in the ceramide domains (GM1-C12:0) for integration into the membrane (Chinnapen et al., 2012). Here we describe the utilization of ER-HA-mNG21-10 which encodes the signal sequence from the ER chaperone BIP at the N-terminal and C-terminal KDEL-motif for localization to the ER. The membrane tethered Sec61β-HA-mNG21-10 construct (mNG21-10 fused with ER-resident protein Sec61β) can also be used to monitor ER retrograde trafficking. Following the addition of CTB-mNG211 to the stably expressing cell lines, the toxin will bind the GM1 receptor and undergo endocytosis and retrograde transport. Once it reaches the ER, the mNG211 fragment of CTB-mNG211 will complement with the mNG21-10 portion of ER-HA-mNG21-10, (alternatively with Sec61β-HA-mNG21-10). The resulting complementation will emit a fluorescence signal and can be assessed by confocal microscopy or flow cytometry to quantify the extent and kinetics of retrograde transport. Kinetic time course experiments on retrograde trafficking across diverse conditions and cell lines can be reliably quantified by flow cytometry. Notably, we obtained high levels of reproducibility in K562 cells with calculated Z-factors of 0.92 and 0.96 for TGN and ER transport, respectively (Luong et al., 2020). A Z-factor > 0.5 is considered ideal for high-throughput screening (Zhang et al., 1999). Hence, this approach can be applied in high throughput for gene or small molecule profiling and discovery to address trafficking at a system-wide level.
Materials and Reagents
Note: With the exception of the listed plasmids, the following reagents can be interchanged with appropriate alternative options.
- Pipette tips (USA Scientific, catalog numbers: 1180-3810 , 1180-1810 , 1180-8810 , 1126-7810 )
- Disposable plastic serological pipets (Corning, catalog numbers: 4487 , 4488 , 4489 , 4490 )
- 6-well clear TC-treated multiple well plates (Corning, CostarTM, catalog number: 3506 )
- 12-well clear TC treated multiple well plate (Corning, CostarTM, catalog number: 3513 )
- 24-well clear TC treated multiple well plate (Corning, CostarTM, catalog number: 3526 )
- 96-well Deep Well Block (Fisher Scientific, catalog number: 12566611 )
- Axygen PCR 8-strip clear tubes (Fisher Scientific, catalog number: 14222251 )
- Coverslips (Fisher Scientific, catalog number: NC9455457 )
- Microscope slides (Fisher Scientific, catalog number: 22034486 )
- Econo-Pac Chromatography Column (Bio-Rad, catalog number: 7321010 )
- SnakeSkin 3.5K MWCO Dialysis Tubing (Thermo ScientificTM, fisher scientific, catalog number: PI88242 )
- HiTrap SP HP column (Sigma-Aldrich, GE Healthcare, catalog number: GE17-1151-01 )
- Inoculating loops (Fisher Scientific, BD DifcoTM, catalog number: 22-031-22 )
- Petri dishes with clear lid (FisherbrandTM, Fisher Scientific, catalog number: FB0875712 )
- Internally threaded cryogenic vials (CorningTM, Fisher Scientific, catalog number: 03-374-059 )
- Seal-Rite® 1.5 ml microcentrifuge tubes (USA Scientific, catalog number: 1615-5510 )
- 15 ml centrifuge tube (Corning®, catalog number: 430052 )
- 50 ml centrifuge tube (Corning®, catalog number: 430897 )
- General purpose syringe BD Luer-LokTM 5 ml (Becton Dickinson, McKesson, catalog number: 309646 )
- Millex-HV syringe filter unit, 45 μm, PVDF, 33 mm, gamma sterilized (Millipore, Millipore Sigma, catalog number: SLHV033RS )
- FalconTM Round-Bottom polystyrene tubes (Corning, Fisher Scientific, catalog number: 352008 )
- BrandTM Plastic Cuvettes (BrandTechTM, Fisher Scientific, catalog number: 759075D )
- SamcoTM Transfer pipettes (Thermo ScientificTM, catalog number 20220S )
- Cell culture treated flask (CorningTM, catalog number: 430639 )
- FalconTM test tube with cell strainer snap cap (CorningTM, Fisher Scientific, catalog number: 08-771-23 )
- Milli-Q water
- CTB pET28a (Addgene, plasmid ID: 141166)
- mNG211-CTA2 pGEXTEV-SUMO (Addgene, plasmid ID: 141167)
- ER-HA-mNG21-10 EF1a-pHAGE-IRES-blasticidin (Addgene, plasmid ID: 141165)
- Sec61β-HA-mNG21-10 Ef1a-pHAGE-IRES-blasticidin (Addgene, plasmid ID: 141213)
- GM1-C12:0 (see Chinnapen et al., 2012)
- Cholera toxin subunit B (recombinant), Alexa FluorTM 488 conjugate (Thermo Fisher, catalog number: C34775 )
- Shuffle® T7 Express Competent E. coli (New England BioLabs, catalog number: C3029J )
- SOC medium (CorningTM, Fisher Scientific, catalog number: 46003CR )
- Miller’s Luria Agar Powder (Research Product International, catalog number: L24022 )
- Miller’s LB Broth Granulated (Fisher BioReagentsTM, Fisher Scientific, catalog number: BP9723 )
- Ampicillin Sodium Salt, Crystalline Powder (GoldBio, catalog number: A301100 )
- Kanamycin (GoldBio, catalog number: K12050 )
- BD DifcoTM Dehydrated Culture Media 2x Yeast extract Tryptone (2x YT) medium (Fisher BioReagentsTM, Fisher Scientific, catalog number: DF0440 )
- Isopropyl-beta-D-thiogalactoside (IPTG) (GoldBio, catalog number: I2481 )
- Trizma® base (Sigma-Aldrich, Millipore Sigma, catalog number: T1503 )
- Sodium chloride ≥ 99.0% (Sigma-Aldrich, Millipore Sigma, catalog number: S9888 )
- TritonTM X-100 (Sigma-Aldrich, Millipore Sigma, catalog number: T8787 )
- Phenylmethanesulfonyl fluoride, PMSF (Thermo ScientificTM, catalog number: 36978 )
- Glutathione Agarose resin (GoldBio, catalog number: G-250 )
- L-Glutathione reduced (Sigma-Aldrich, Millipore Sigma, catalog number: G4251 )
- ULP1 protease (produced in-house, Addgene, plasmid ID 64697)
- Sodium phosphate dibasic, Na2HPO4 (Sigma, catalog number: S9763 )
- 4-20% Mini-Protean® TGXTM Precast Gels, 10-well, 50 μl (Bio-Rad, catalog number: 4561094 )
- Precision Plus unstained protein standard (Bio-Rad, catalog number: 161-0363 )
- 10x Tris Glycine SDS PAGE Buffer (national diagnostics, catalog number: EC-870 )
- Ez-RunTM Protein Gel staining Solution (Fisher BioReagentsTM, Fisher Scientific, catalog number: BP36201 )
- Liquid nitrogen
- Absolute ethanol (Pharmco, catalog number: 111000200CSPP )
- RPMI Medium 1640, GlutaMAXTM-I (Thermo Fisher Scientific, catalog number: 61870 )
- Fetal Bovine Serum, qualified, One ShotTM format, heat inactivated (Thermo Fisher Scientific, catalog number: A3160602 )
- Fetal Bovine Serum, fatty acid free (Sigma-Aldrich, catalog number: A6003-10G )
- Penicillin-Streptomycin Solution (CorningTM, Fisher Scientific, catalog number: MT30002CI )
- Trypsin-EDTA (0.25%), phenol red (GibcoTM, Fisher Scientific, catalog number: 25-200-072 )
- Cell culture phosphate buffered saline 1x (CorningTM, Fisher Scientific, catalog number: MT21040CV )
- DMEM with L-glutamine, 4.5 g/L glucose and sodium pyruvate (CorningTM, Fisher Scientific, catalog number: MT10013CV )
- Lentivirus packaging plasmids psPAX2 (Addgene, plasmid ID: 12260 )
- Lentivirus packaging plasmids pVSVG (Addgene, plasmid ID: 8454)
- Polyethylenimine, PEI (Fisher Scientific catalog number: NC1014320 )
- Polybrene (Santa Cruz Biotechnology, catalog number: sc134220 )
- Blasticidin (GoldBio, catalog number: B800 )
- ER-mCherry plasmid (available on request)
- Anti-HA antibody (Sigma-Aldrich, Millipore Sigma, catalog number: 11867423001 )
- Anti-Golgin97 (Thermo Fisher, catalog number: A21270 )
- Appropriate secondary antibody (Invitrogen, catalog numbers: A21247 and A11001 )
- 20% paraformaldehyde aqueous solution (Electron Microscopy Science, Fisher Scientific, catalog number: 15713S )
- Saponin (Sigma-Aldrich, Millipore Sigma, catalog number: 47036 )
- Normal Goat serum (Sigma-Aldrich, Millipore Sigma, catalog number: G902310 )
- Coomasie blue (Fisher BioreagentsTM, EZ-runTM, catalog number: BP36201 )
- Collagen (Sigma-Aldrich, catalog number: C7661 )
- FluorSave mountant (Millipore, catalog number: 345789 )
- 100 mg/ml (1,000x) ampicillin stock (see Recipes)
- 50 mg/ml (1,000x) kanamycin stock (see Recipes)
- LB agar plate (see Recipes)
- LB media (see Recipes)
- 2x YT media (see Recipes)
- 1 M IPTG stock solution (see Recipes)
- 50% glycerol (see Recipes)
- Triton X-100 lysis buffer (see Recipes)
- Binding buffer (see Recipes)
- Elution buffer (see Recipes)
- Dialysis Buffer (see Recipes)
- Buffer A (see Recipes)
- Buffer B (see Recipes)
- K562 cell media (see Recipes)
- HEK 293T and COS7 cell media (see Recipes)
- Blasticidin stock (see Recipes)
- 4% paraformaldehyde (PFA) (see Recipes)
- 0.2% saponin (see Recipes)
- FACS Buffer (see Recipes)
Equipment
Note: The following equipment brand and manufacturers are suggestions and can be interchanged with appropriate alternative options.
- Micropipettes (Gilson, catalog number: F167360 )
- Pipette aid (Drummond Scientific, catalog number: 4-000-102 )
- Appropriate personal protective equipment
- 250 ml conical flask (Sigma, catalog number: CLS5100250 )
- 2 L conical flask (Sigma, catalog number: CLS51002L )
- Magnetic stirring bar (Fisher Scientific, FisherbrandTM, catalog number: 14-513-52 )
- Magnetic stirrer (Thermolyne Nuova, catalog number: Z110930 )
- Vortex mixer (Fisher Scientific, Vortex-GenieTM 2, catalog number: 15557335 )
- Heat block (Benchmark Scientific, catalog number: BSW1500 )
- Autoclave
- Tabletop incubator (Barnstead Lab Line 100)
- Centrifuge capable of spinning 500 ml bottles (Thermo Scientific, Sorvall Lynx 4000, catalog number: 75006580 )
- 500 ml centrifuge bottles (Fisher Scientific, catalog number: 05-564-2 )
- Emulsiflex C5 homogenizer (Avestin, catalog number: EF-C5 )
- Tabletop microcentrifuge (Eppendorf, catalog number: 12843222 )
- Platform Shaker at 4 °C (Orbital Shaker)
- Stericup-GP Vacuum Filtration System (Millipore, Millipore Sigma, catalog number: SCGPU05RE )
- NanoDrop 2000 (Thermo ScientificTM, catalog number: ND-2000 )
- NGC QuestTM 10 Plus Chromatography system (Bio-Rad, catalog number: 7880003 )
- Azure C300 gel imaging system (Azure biosystem)
- Cell culture incubator (Thermo Fisher Scientific, FormaTM Series II, catalog number: 3110 )
- Biosafety cabinet (Labcult)
- Water bath
- Spectrophotometer
- pH meter
- Electrophoresis chamber (Bio-Rad, Mini-PROTEAN tetra)
- Powerpack (Bio-Rad, PowerpacTM universal power supply, catalog number: 1645070 )
- -20 °C freezer
- -80 °C freezer
- BD LSRFortessaTM Flow cytometer system (BD biosciences)
- Spinning disk confocal head coupled to an inverted Zeiss Axiovert 200M microscope
Software
Note: Equivalent software can be used.
- ChromlabTM Software 6.0 (Bio-Rad, https://www.bio-rad.com/en-us/product/chromlab-software?ID=MFCVPXIVK)
- GraphPad Prism Software version 8.0 (https://www.graphpad.com)
- BD FACSDivaTM Software (BD, https://www.bdbiosciences.com/en-us/instruments/research-instruments/research-software/flow-cytometry-acquisition/facsdiva-software)
- FlowJoTM Software v10.6.2 (BD, https://www.flowjo.com)
- Excel (Microsoft Corporation, https://www.microsoft.com/Microsoft/Excel)
- Zeiss Axiovert 200M microscope, spinning disk confocal head
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Simpson, M. S., Lencer, W. I. and Luong, P. (2020). A Quantitative Single-cell Flow Cytometry Assay for Retrograde Membrane Trafficking Using Engineered Cholera Toxin. Bio-protocol 10(15): e3707. DOI: 10.21769/BioProtoc.3707.
分类
细胞生物学 > 基于细胞的分析方法 > 流式细胞术
细胞生物学 > 基于细胞的分析方法 > 内吞作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link