- EN - English
- CN - 中文
Generation and Testing of Fluorescent Adaptable Simple Theranostic (FAST) Proteins
FAST蛋白的生成与检测
发布: 2020年07月05日第10卷第13期 DOI: 10.21769/BioProtoc.3696 浏览次数: 8794
评审: Hongwei HanKate HannanAnonymous reviewer(s)
Abstract
This protocol provides a step-by-step method to create recombinant fluorescent fusion proteins that can be secreted from mammalian cell lines. This builds on many other recombinant protein and fluorescent protein techniques, but is among the first to harness fluorescent fusion proteins secreted directly into cell culture supernatant. This opens new possibilities that are not achievable with proteins produced in bacteria or yeast, such as direct use of the fluorescent protein-secreting cells in live co-culture assays. The Fluorescent Adaptable Simple Theranostic (FAST) protein system includes a histidine purification tag and a tobacco etch virus (TEV) cleavage site, allowing the purification tag and fluorescent protein to be removed for therapeutic use. This protocol is split into five parts: (A) In silico characterization of the gene-of-interest (GOI) and protein-of-interest (POI); (B) design of the expression vector; (C) assembly of the expression vector; (D) transfection of a eukaryotic cell line with the expression vector; (E) testing of the recombinant protein. This extensive protocol can be completed with only polymerase chain reaction (PCR) and cell culture training. Additionally, each part of the protocol can be used independently.
Keywords: Fluorescent (荧光)Background
Recombinant proteins are key tools for many basic research and biomedical fields. Production of recombinant proteins generally entails design and assembly of expression vectors followed by production of the recombinant protein in prokaryotic or eukaryotic cells. Expression of proteins in eukaryotic cells is often preferred when post-translational modifications, such protein glycosylation, is important for downstream functional testing. Many excellent protocols are available for aspects of the full recombinant protein production cycle (Benson et al., 2013; Flies et al., 2020), but few are available the provide step-by-step details for the entire production and functional testing process. This protocol can be adapted to produce species-specific recombinant proteins with and without a fluorescent reporter protein fused to a protein of interest (POI). It can also be used for creating vectors for non-secreted proteins (e.g., cell surface proteins). The methods can be used for most eukaryotic species, but we have focused on a single gene from the Tasmanian devil (Sarcophilus harrisii) for illustrative purposes. This protocol will result in a recombinant protein that includes the extracellular domain (ECD) of the CD200 (aka OX-2) protein that is fused directly to a fluorescent reporter protein. CD200 is an immune checkpoint protein that is highly expressed on several types of cancer. This protein will be secreted from mammalian cells after transfection and can be used directly from supernatant or purified for downstream use. An overview of the complete protocol can be seen in Figure 1 (Flies et al., 2020).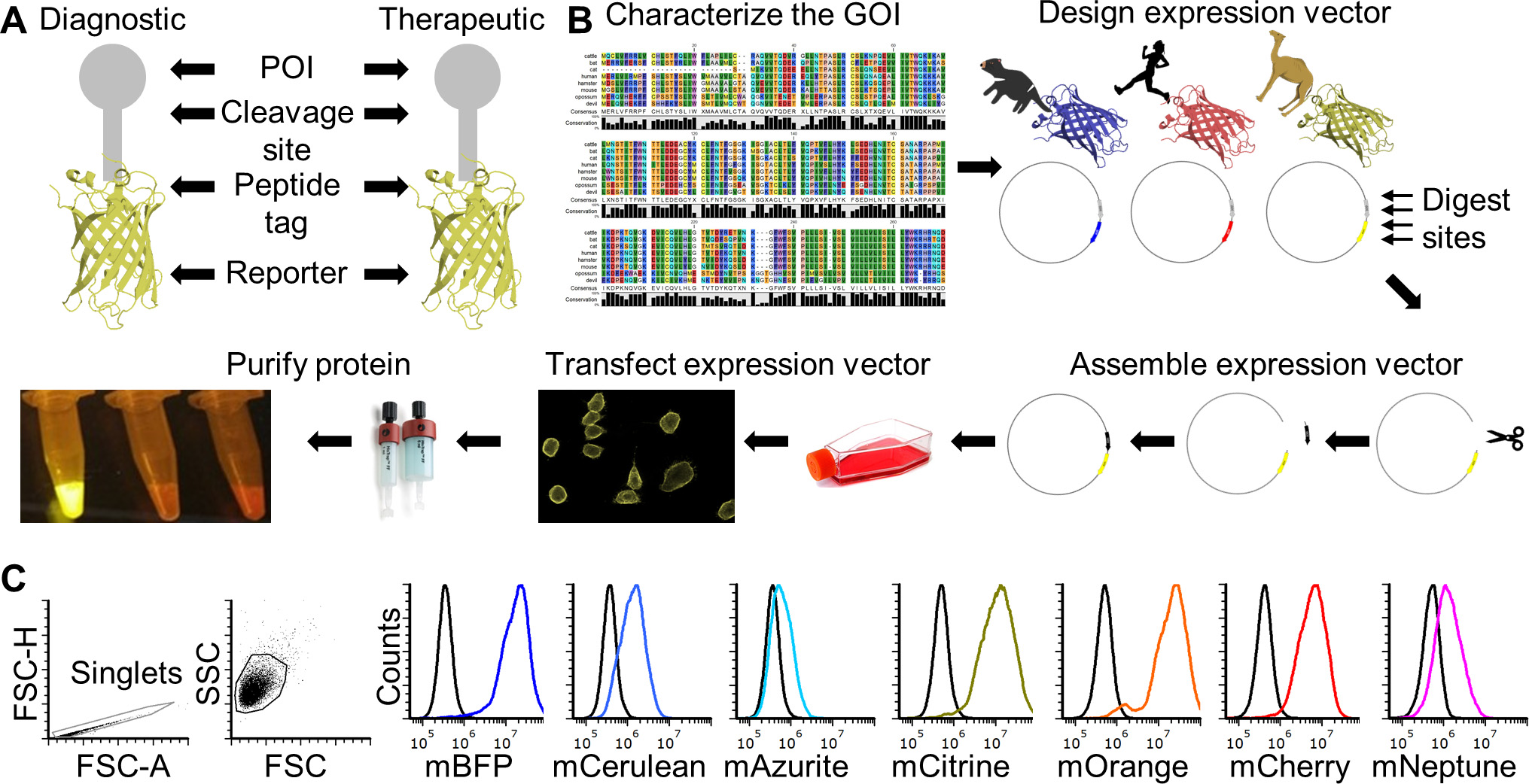
Figure 1. FAST protein schematic and initial testing. A. Schematic diagram of FAST protein therapeutic and diagnostic (i.e., theranostic) features. B. Graphic overview of FAST protein system including key steps: (1) characterize gene-of-interest (GOI) in silico; (2) design expression vectors; (3) digest FAST base vectors and insert alternative GOIs or colors; (4) transfect expression vectors into mammalian cells and monitor using fluorescent microscopy or flow cytometry; (5) purify the protein using 6xHistidine tag, visualize fluorescent color to show protein is in-frame and correctly folded. Image of microfuge tubes shows 100 μl of mCitrine, mOrange, and mCherry FAST proteins (1 mg/ml) excited with blue light with amber filter. Full protocols for vector construction and protein testing are available in the Supplementary Materials. C. Results of flow cytometry binding assay using Tasmanian devil 41BB (aka TNFRSF9, CD137) FAST proteins and cell lines expressing 41BB ligand (aka TNFSF9, CD137L). The colored lines in the histograms show binding of devil 41BB fused to mTagBFP, mCerulean3, mAzurite, mCitrine, mOrange, mCherry, or mNeptune2 to Chinese hamster ovary (CHO) cells transfected with devil 41BBL, and the black lines show binding to untransfected CHO cells. Figure reprinted from Flies et al. (2020) under CC BY-NC license.
The recombinant protein construct includes a linker protein with three additional features (Figure 1A). First is a TEV cleavage site, which allows the protein to be cleaved to separate the POI and the fluorescent reporter protein. Second is a rigid linker protein that provides additional separation of the POI and the reporter (i.e., so the two proteins do not interact with each other). Third is a 6x histidine (6xHis) tag that allows for easy purification from cell culture supernatant.
The protocol here describes how to make Tasmanian devil CD200-mTagBFP and CD200-mOrange Fluorescent Adaptable Simple Theranostic (FAST) proteins, but can be adapted to make most other type I transmembrane proteins and secreted proteins (e.g., cytokines). Simply replace the CD200 coding sequence with a different gene of interest (GOI) and repeat the step-by-step protocol. We have also used the expression vectors to produce type II transmembrane proteins (see definitions below for type I and II proteins).
We have made a spreadsheet available that has templates for performing each of the major experiments necessary to complete this protocol. The spreadsheet contains a tab with the list of reagents, a tab for recipes, and a tab for each experiment. Every experiment in our lab is given a unique ID based on the person performing the experiment. For example, the first experiment done by Andrew S. Flies would be exp_ASF_1. Each new lab member has their own three-letter code. We have titled the experiments in the accompanying spreadsheet as exp_ID_1.
FAST protein experiment templates from Flies et al. (2020)–Science Advances DOI: 10.1126/sciadv.aba5031.
Materials and Reagents
- 0.22 μm PVDF syringe filter (Merck/Millipore, catalog number: SLGV033RS )
- 20 ml syringe (any supplier)
- 6-well plates, tissue culture treated (any supplier)
- 96-well plate, flat-bottom, tissue culture treated (any supplier)
- 96-well plate, U-bottom, tissue culture treated (any supplier)
- Amicon Ultra centrifugal filter units, Ultra-15, MWCO 10 kDa (Merck/Millipore, catalog number: UFC901008 )
- Bacterial spreader (Merck/Sigma, catalog number: Z723193-500EA )
- Flask, T175, tissue culture treated (T25, T75, T175, any supplier)
- PCR tubes (any supplier)
- Petri dishes, 100 mm (any supplier)
- pH meter or pH strips (any supplier)
- Pipet tips (unfiltered): 10, 200 and 1,000 μl (any supplier)
- Pipet tips (filtered): 10, 200 and 1,000 μl (any supplier)
- HisTrap excel columns (5 x 1 ml) (GE Life Sciences, catalog number: 17371205 )
- CHO-K1 cells (ATCC, catalog number: CCL-61 )
- E. coli DH5α competent cells (New England Biolabs, comes with NEBuilder kit)
- Agarose (any supplier)
- Agencourt CleanSEQ–Dye Terminator Removal (Beckman Coulter, catalog number: A29151 )
- Antibiotic Antimycotic Solution (100x) (Merck/Sigma, catalog number: A5955 )
- Big Dye Terminator kit (Thermo Fisher, catalog number: 4337455 )
- EX-CELL CHO protein free media (Merck/Sigma, catalog number: 14361C-1000ML )
- ExpiCHO expression system (optional for high yield protein production) (Thermo Fisher, catalog number: A29133 )
- Formalin solution, neutral buffered, 10% (Merck/Sigma, catalog number: HT501128-4L )
- Hyrdochloric acid (HCl) 12 M or higher (any supplier)
- Hygromycin (Merck, catalog number: H0654-1G )
- NEBuilder HiFi DNA assembly cloning kit (New England Biolabs, catalog number: E5520S )
- NotI-HF (New England Biolabs, catalog number: R3189S )
- Nucleic acid gel stain (any supplier)
- OneTaq® Hot Start Quick-Load® 2x Master Mix with Standard Buffer (New England Biolabs, catalog number: M0488L )
- Plasmid miniprep kit (Machery-Nagel, catalog number: 740499.5 0)
- Polyethylenimine (PEI) (linear, MW 25,000) (Polysciences, catalog number: 23966-2 )
- Pur-A-LyzerTM Mega Dialysis Kit (Merck/Sigma, catalog number: PURG12020-1KT )
- Q5® Hot Start High-Fidelity 2x Master Mix (New England Biolabs, catalog number: M0494L )
- RPMI-1640 medium (Merck/Sigma, catalog number: R7509 )
- SmaI (New England Biolabs, catalog number: R0141S )
- SOC media (New England Biolabs, comes with NEBuilder kit)
- Sodium hydroxide (NaOH) 10 M or higher (any supplier)TrypLE Express enzyme (Thermo Fisher, catalog number: 12604039 )
- Water (e.g., Milli-Q deionized-distilled) (any supplier)
- NaCl
- KCl
- Na2HPO4
- KH2PO4
- Tris base
- FBS (heat inactivated)
- Glutamine
- 2-ME
- HEPES
- DMEM
- FCS
- Sodium-pyruvate
- DMSO
- Tryptone
- Yeast extract
- KHCO3
- Glycerol
- Glycine
- Bromophenol blue
- 1x Phosphate-buffered saline pH 7.4 (see Recipes)
- 10x Phosphate-buffered saline pH 7.4 (see Recipes)
- Tris-buffered saline (TBS) pH 7.5 (see Recipes)
- 20% ethanol (see Recipes)
- 70% ethanol (see Recipes)
- 1 M HCl (see Recipes)
- 1 M NaOH (see Recipes)
- Complete RPMI with 10% fetal bovine serum (cRF10) (see Recipes)
- Complete RPMI with 5% fetal bovine serum (cRF5) (see Recipes)
- Complete RPMI without fetal bovine serum (cRF0) (see Recipes)
- Complete DMEM with 10% FBS (cDF10) (see Recipes)
- Complete IMDM with 20% FBS (cIF20) for hybridomas (see Recipes)
- 2-mercaptoethanol (2-ME) (Merck/Sigma, catalog number: M3148 ) (see Recipes)
- 2x cell freezing media (see Recipes)
- Ampicillin stock (see Recipes)
- Luria broth (LB medium) (see Recipes) or (Merck/Sigma, catalog number: L3522 )
- Luria broth (LB) agar (see Recipes) or (Merck/Sigma, catalog number: L3147 )
- LB medium (low salt) (see Recipes)
- LB agar (low salt) (see Recipes)
- Bacterial freezing media (2x) (see Recipes)
- 1.5 M Tris pH 8.8 (see Recipes)
- 1 M Tris pH 6.8 (see Recipes)
- 1 M Tris pH 9.0 (see Recipes)
- Ammonium chloride (block lysosomal degradation) (see Recipes)
- Chloroquine diphosphate (Merck/Sigma, catalog number: C6628 ) (see Recipes)
- RBC lysis buffer (see Recipes)
- 10% sodium azide (see Recipes)
- 4% paraformaldehyde (see Recipes)
- Flow cytometry wash buffer with sodium azide, without EDTA (aka FACS buffer) (see Recipes)
- Flow cytometry wash buffer with sodium azide and EDTA (aka FACS buffer) (see Recipes)
- Flow cytometry fixation buffer (see Recipes)
- 2x Laemmli buffer (see Recipes)
- 6x SDS reducing buffer (see Recipes)
- 0.01 M Tris-HCl (see Recipes)
- Tris-Glycine SDS running buffer (see Recipes)
- 0.15 M NaCl (see Recipes)
- 0.5 M EDTA (pH 8.0) (see Recipes)
- 50x TAE (see Recipes)
- Glycerol & bromophenol blue gel loading buffer (6x) (see Recipes)
- ELISA coating buffer (see Recipes)
- ELISA Wash buffer (see Recipes)
- D-luciferin potassium salt (see Recipes)
- Lysis buffer for RNA extraction (see Recipes)
- BDT sequencing buffer (see Recipes)
- Immunofluorescence blocking buffer (see Recipes)
- HisTrap Excel Reagents (see Recipes)
5 M imidazole
10x equilibration buffer
Equilibration buffer
Wash buffer
Elution buffer
Equipment
- 1 L beaker (any supplier)
- Magnetic stir bar (any supplier)
- ÄKTA start protein purification system (GE Life Sciences, catalog number: 29022094-ECOMINSSW )
Note: A simple peristaltic pump or gravity flow columns can be used instead to save cost. - Access to a DNA sequencing machine (any supplier)
- Automated cell counter or haemocytometer (any supplier)
- Bacteria shaker at 37 °C (any supplier)
- Biosafety cabinet for sterile cell culture (BSC) (any supplier)
- Cell culture incubators with 5% CO2 and 37 °C (any supplier)
- Centrifuge, refrigerated is preferred but not necessary (any supplier)
- Computer (any supplier)
- Flow cytometer (any supplier)
- Gel electrophoresis equipment (any supplier)
- Gel Documentation System (any supplier)
- Magnetic stirrer (any supplier)
- Pipet controller (aka Pipet-Aid, pipettor) (any supplier)
- Pipets (10 μl, 100 μl, 200 μl, 1,000 μl) (any supplier)
- Thermocycler (any supplier)
- Spectrophotometer (any supplier)
Software
- NCBI BLASTN for sequence comparison (free use online; free download available: https://blast.ncbi.nlm.nih.gov/Blast.cgi)
- SnapGene (free sequence viewer use online; purchase recommended for plasmid construction, SnapGene: https://www.snapgene.com/)
Note: Software for designing plasmids and aligning sequencing results. Other options are available, but this is the best in our opinion. - CLC Sequence Viewer (CLC sequence viewer is free; CLC Main Workbench, Qiagen available here)
- Phobius (free use online: http://phobius.sbc.su.se/)
Online algorithm for determine the location of SigP (Signal peptide), ECD, TMD (Transmembrane domain), and Intracellular domain, (ICD) for the protein-of-interest. - TMHMM server (free use online: https://services.healthtech.dtu.dk/service.php?TMHMM-2.0)
Prediction of transmembrane helices in proteins. Should produce results that nearly match Phobius. - SignalP (free use online: http://www.cbs.dtu.dk/services/SignalP/)
The SignalP 5.0 server predicts the presence of signal peptides and the location of their cleavage sites in proteins from Archaea, Gram-positive Bacteria, Gram-negative Bacteria and Eukarya. - Simple Modular Architecture Research Analysis (SMART) (free use online: http://smart.embl-heidelberg.de/)
Identifies key structural domains in proteins. Complements Phobius and TMHMM analysis. - Eukaryotic Linear Motif (ELM) resource (free use online: http://elm.eu.org//index.html)
Annotation and detection of eukaryotic linear motifs (i.e., regions of the protein sequence that have known functions). - Webcutter (free use online: http://heimanlab.com/cut2.html)
Tool for finding potential restriction sites in a DNA sequence by introducing silent mutations that do not change the protein sequence. This is used to introduce restriction sites in the plasmid during construction that can then be used for downstream modifications of the plasmid. - Russel Lab (free use online: http://www.russelllab.org/aas/)
Note: If you need to change an amino acid to introduce a restriction site, then check the amino acid proper ties and consequences of substitutions to see which amino acid is the best substitution.
Databases (free to use online)
- Genbank (https://www.ncbi.nlm.nih.gov/genbank/) (Benson et al., 2013)
Sequence database maintained by the National Center for Biotechnology Information (NCBI), National Library of Medicine (NLM), and National Institutes of Health (NIH) - Ensembl (http://ensembl.org/) (Zerbino et al., 2018)
Sequence database maintain by the European Molecular Biology Laboratory (EMBL) - UniProt (https://www.uniprot.org/) (Consortium, T. U. 2018)
This should be a first stop for basic understanding of any protein of interest. Annotations for human and mouse genes are very detailed and in most cases the proteins for other species have similar features (e.g., locating the extracellular and transmembrane domains within the protein). Commercial suppliers are also useful for cross-referencing UniProt and your own protein analysis results (e.g., RnD Systems).
Note: We have found that for species with limited information (e.g., Tasmanian devils), a de novo transcriptome assembly is extremely useful. The initial genome assemblies for many species are incomplete and/or inaccurate, so Genbank and Ensembl may not contain the correct sequence for your GOI. A de novo assembly of RNA sequencing data can provide accurate full gene transcripts that can be used to cross-check with Genbank and Ensembl sequences. Furthermore, the use of a de novo transcriptome assembly allows this protocol to be applied in species where reference genome assemblies are not available. RNA sequencing data can obtain with single-read or paired-end protocols, although paired-end data is recommended for greater sequence confidence. A transcriptome from peripheral blood cells, spleen, or lymph node should yield most of the immune system related genes. A de novo transcriptome assembly requires deeper bioinformatics skills than the rest of this protocol, so for teams without this expertise we recommend finding an experienced collaborator in the first case and then developing your own skills if needed. Please contact Dr Andrew Flies @WildImmunity for help finding a collaborator with the necessary skills.
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Flies, A. S., Darby, J. M., Murphy, P. R., Pinfold, T. L., Patchett, A. L. and Lennard, P. R. (2020). Generation and Testing of Fluorescent Adaptable Simple Theranostic (FAST) Proteins. Bio-protocol 10(13): e3696. DOI: 10.21769/BioProtoc.3696.
- Flies, A. S., Darby, J. M., Lennard, P. R., Murphy, P. R., Ong, C. E. B., Pinfold, T. L., De Luca, A., Lyons, A. B., Woods, G. M. and Patchett, A. L. (2020). A novel system to map protein interactions reveals evolutionarily conserved immune evasion pathways on transmissible cancers. Science Advances 6(27): eaba5031.
分类
癌症生物学 > 肿瘤免疫学 > 免疫学试验 > 蛋白质分析
分子生物学 > 蛋白质 > 蛋白质-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link