- EN - English
- CN - 中文
Generation of T cells from Human and Nonhuman Primate Pluripotent Stem Cells
从人类和非人类灵长类动物多能干细胞中产生T细胞
发布: 2020年07月05日第10卷第13期 DOI: 10.21769/BioProtoc.3675 浏览次数: 7273
评审: Sudan PuriAnonymous reviewer(s)

相关实验方案
使用康可藻红素刺激冷冻保存的猪外周单个核细胞进行增殖检测,并结合FCS ExpressTM 7.18软件分析
Marlene Bravo-Parra [...] Luis G. Giménez-Lirola
2025年06月05日 2614 阅读
Abstract
Pluripotent stem cells (PSCs) have the potential to provide homogeneous cell populations of T cells that can be grown at a clinical scale and genetically engineered to meet specific clinical needs. OP9-DLL4, a stromal line ectopically expressing the Notch ligand Delta-like 4 (DLL4) is used to support differentiation of PSCs to T-lymphocytes. This article outlines several protocols related to generation of T cells from human and non-human primate (NHP) PSCs, including initial hematopoietic differentiation of PSC on OP9 feeders or defined conditions, followed by coculture of the OP9-DLL4 cells with the PSC-derived hematopoietic progenitors (HPs), leading to efficient differentiation to T lymphocytes. In addition, we describe a protocol for robust T cell generation from hPSCs conditionally expressing ETS1. The presented protocols provide a platform for T cell production for disease modeling and evaluating their use for immunotherapy in large animal models.
Keywords: Human pluripotent stem cells (人类多能干细胞)Background
T lymphocyte (T cells) play a key role in cell-mediated immune responses and are involved in monitoring and killing tumor cells. Throughout the last decades, several strategies have been developed to redirect, culture and/or enhance T lymphocytes against cancer (Houot et al., 2015; June et al., 2018) and utilize them for T cell-based adoptive immunotherapies. Recent clinical trials have shown outstanding outcomes in relapsed and refractory lymphoma patients treated with chimeric antigen receptor (CAR)-T cells (Riviere and Sadelain, 2017).
Human pluripotent stem cells (hPSCs), including embryonic (hESCs) and induced (hiPSCs), provide a promising resource to produce T cells for adoptive cellular immunotherapies, which can be coupled with genetic engineering technologies to generate off-the-shelf supplies of CAR T cells. In addition, generating hPSCs from antigen (Ag)-specific cytotoxic T lymphocytes (CTLs) and redifferentiating them into functional CTLs could enable the scalable production of rejuvenated CTLs (Minagawa and Kaneko, 2014; Kaneko, 2016). Several reports have demonstrated T cell generation from hPSCs (Nishimura et al., 2013; Vizcardo et al., 2013) and the feasibility of hiPSC based CAR T cell therapies (Themeli et al., 2013). However, there is still a need to improve the efficacy of T cell generation and expansion from hPSCs. In addition, further advances in hPSC-based T cell therapies will require their preclinical evaluation in large animal models. Since macaques are physiologically and immunologically similar to humans, including possessing orthologous MHC genes (Adams et al., 2001), and similarities in killer cell immunoglobulin-like receptors (KIR) with humans (Bimber et al., 2008; Parham et al., 2010), nonhuman primates (NHPs) will be the most appropriate model to address the therapeutic efficacy, safety and immunogenicity of PSC-derived T cells.
Here, we describe an improved method for the derivation of T cells from human and NHP-PSCs with a higher efficiency and shorter time (as soon as 3 weeks) than existing protocols. Differentiation of T cells from hPSCs involves two major steps: induction of hematopoietic progenitor cells (HPs) from hPSCs and their subsequent differentiation into T cells. Our lab previously reported well-established protocols on the induction of hematopoietic lineages from hPSCs on OP9 feeders and in defined feeder- and serum-free conditions (Vodyanik et al., 2005; Vodyanik and Slukvin, 2007; Uenishi et al., 2014). We showed that hemogenic progenitors from different stages of differentiation or different sources were cocultured on OP9-DLL4 to differentiate into T cells (Kumar et al., 2019b). We have also reported a protocol for the induction of hematopoietic lineages from NHP-PSCs (D'Souza et al., 2016). T cells differentiation from both hPSCs or NHP-PSCs proceeds through a CD5+CD7+ progenitor stage that eventually transitions into CD8+CD4+ double-positive cells. Altogether, the protocol used for the PSC-derived T cells presents a platform for T cell production to evaluate their utility for adoptive immunotherapies and preclinical testing in large animal models.
Related Information
Hematopoietic differentiation from human PSCs in an OP9 co-culture system
Hematopoietic differentiation of hPSCs on mouse stromal OP9 feeders is performed in serum-containing medium without addition of any cytokines (Vodyanik et al., 2005). In this system, hPSCs undergo stepwise progression toward APLNR+PDGFRα+ primitive posterior mesoderm with hemangioblast colony forming cells (HB-CFCs) that reflects primitive hematopoiesis, KDRhiPDGFRαlo/-VEC- hematovascular mesodermal progenitors with definitive hematopoietic potential, immature VE-cadherin (VEC)+CD43-CD73- HE, which specify into DLL4+ arterial hemogenic endothelium (HE) with definitive hematopoietic potential, and DLL4- non-arterial-type HE with mostly primitive hematopoietic potential; and CD34+CD43+ hematopoietic progenitors (HPs) that include CD43+CD235a+CD45+/- HPs, enriched in erythromegakryocytic progenitors and CD43+CD235a/41a- multipotent HPs with a lin-CD34+CD90+CD38-CD45RA- hematopoietic stem progenitor cells phenotype, and lymphomyeloid potential (Vodyanik et al., 2006; Choi et al., 2009a and 2009b; Choi et al., 2012; Kumar et al., 2019a and 2019b; Uenishi et al., 2018) (Figure 1). CD43+ HPs generated in this coculture can be collected on days 8-9 of differentiation and subsequently cultured on OP9-DLL4 in lymphoid conditions to generate T cells (Kumar et al., 2019b). We also demonstrated that T cells can be generated directly from definitive hemogenic progenitors collected from earlier stages of development (hematovascular mesoderm or HE stage) and cultured in lymphoid conditions on OP9-DLL4 (Kumar et al., 2019b).
Hematopoietic differentiation from human PSCs in a chemically defined system
We have reported defined feeder- and serum-free conditions for generation of blood from hPSCs in chemically defined conditions. In this differentiation system, hPSCs follow stages of development similar to those described in hPSCs cocultured on OP9 feeders, including the formation of VE-Cadherin+CD73-CD235a/CD43- HE and CD34+CD43+ HPs with myeloid and T lymphoid potential (Uenishi et al., 2014) (Figure 1). We typically use collagen IV coated plates for differentiation in defined conditions to reduce cost. However, more expensive matrix Tenascin C can be used instead of collagen IV to improve hematopoietic differentiation and promote development of HPs with T cell potential in defined conditions (Uenishi et al., 2014).
Genetic engineering of Doxycycline-inducible iETS1 hPSCs
We have recently reported a protocol for the generation of conditional gene expression of ETS1 under tetracycline responsive element (TRE) promoter along with M2rtTA (reverse tetracycline transactivator) introduced into hPSCs using PiggyBac transposons (Jung et al., 2016; Park et al., 2018a and 2018b). In one vector ETS1 is downstream from the TREtight promoter, along with the zeocin resistance gene driven by the EF1α promoter, subcloned between the ends of 2 ITRs of the transposon vector. For easy detection of transgene expression upon addition of doxycycline to the culture, ETS1 is linked with Venus through a 2A self-cleaving peptide sequence. The second vector has the M2rtTA promoter linked with a puromycin resistance gene through a 2A peptide sequence subcloned between the ends of 2 ITRs. Using 2 different antibiotic genes facilitates the selection of clones that incorporate both vectors in a single step. The use of a two-vector system allows flexibility to adjust the TRE/M2rtTA ratio to achieve robust doxycycline dependent gene expression in hPSCs while limiting transgene leakage. hPSCs are cultured and then transfected on matrigel plates in mTeSR1 medium. Single hPSC colonies can be picked up from low-density cultures of cell populations (Park et al., 2018a and 2018b).
T cell production from iETS1 hPSCs
We have demonstrated that T cell production from hESCs can be increased through activation of the arterial program at the mesodermal stage of development by overexpression of the transcription factor ETS1. Hemogenic progenitors generated following induction of ETS1 were more than 100-fold enriched in T cell precursors as compared to control (Park et al., 2018b). Doxycycline treatment of differentiation cultures from days 2 to 6, enhances the generation of CD34+ HPs with lymphoid potential. HPs collected from day 9 of differentiation cultures in the presence of doxycycline can subsequently be differentiated into T cells in coculture with OP9-DLL4. Although T cell cultures from DOX- and DOX+ conditions generate a similar percentage of CD5+CD7+ and CD4+CD8+ T cells, total numbers of T lymphocytes produced per 104 CD43+ cells from DOX-treated cultures are dramatically (> 8-fold) greater.
Hematopoietic differentiation of NHP-PSCs on OP9 coculture
Although the defined serum and feeder-free differentiation system described above works well with hPSCs, it does not support efficient generation of CD34+CD43+ HPs with T cell potential from NHP-PSCs. That is why we recommend to culture on OP9 feeders to induce efficient production of lymphoid progenitors from NHP-PSCs. OP9 coculture supports blood production from different NHP species, including ESCs and iPSCs derived from rhesus and cynomolgus macaques. However, in contrast to human PSCs, NHP-PCS/OP9 cocultures require addition of GSK3β inhibitor and VEGF to promote hemogenic mesoderm development and human hematopoietic cytokines to support blood development (D'Souza et al., 2016) (Figure 2). Similar to human, NHP-PSC-derived HPs can be differentiated into T cells in coculture with OP9-DLL4 (D'Souza et al., 2016).
Materials and Reagents
- Cell lines
- hESC WA01 and WA09 (WiscBank, WiCell, Madison, WI)
- Transgene-free iPSCs, DF-19-9-7T and 4-3-7T33 (WiscBank, WiCell, Madison, WI)
- Mouse embryonic fibroblasts (MEFs, WiCell, Madison, WI)
- Non-human primate PSCs (NHP-iPSCs), RhF5-iPS 19.1, Cy.F 3L iPS, and Cy0669#1 iPS (D'Souza et al., 2016)
- OP9 mouse bone marrow stromal cell line (Provided by Dr. Toru Nakano, Osaka University, Japan)
- Lenti-XTM 293T Cell Line (Clontech, catalog number: 632180 )
- Reagents
- α-MEM basal medium, powder (Life Technologies, catalog number: 12000-022 )
- DMEM/nutrient mixture F-12, powder (Life Technologies, catalog number: 12400-024 )
- DMEM powder (Life Technologies, catalog number: 12100-046 )
- Iscove's Modified Dulbecco's Medium (IMDEM), powder (Life Technologies, catalog number: 122-00036 )
- Ham's F-12 Nutrient Mixture (F12), powder (Life Technologies, catalog number: 217-00075 )
- Chemically Defined Lipid Concentrate (CDLC) (Life Technologies, catalog number: 119-050-31 )
- GlutaMax (Life Technologies, catalog number: 35050-061 )
- Non-essential Amino Acids (NEAA) (Life Technologies, catalog number: 11140-076 )
- DPBS powdered, without calcium and magnesium (Life Technologies, catalog number: 21600-044 )
- EDTA 0.5 M, pH 8.0 (Life Technologies, catalog number: 15575-038 )
- KnockOut Serun Replacement (KOSR) for hPSCs (Life Technologies, catalog number: 10828-023 )
- TrypLe Select (Life Technologies, catalog number: 12563-029 )
- Collagenase type IV (Life Technologies, catalog number: 17104-019 )
- Puromycin (Life Technologies, catalog number: A1113803 )
- Primate ES cell medium (REPROCELL, catalog number: RCHEMD001 )
- mTeSR1 defined feeder-free medium (StemCell Technologies, catalog number: 0 5850 )
- TeSR-E8 (E8) medium (Stem Cell Technologies, catalog number: 0 5990 )
- Vitronectin XFTM (VTN) (Stem Cell Technologies, catalog number: 0 7180 )
- Sodium Selenite (Millipore Sigma, catalog number: S5261 )
- Tenascin C (Millipore Sigma, catalog number: CC065 )
- Collagen IV (CollV) (Millipore Sigma, catalog number: C5533 )
- Acetic Acid (Millipore Sigma, catalog number: 537020 )
- Holo-transferrin (Millipore Sigma, catalog number: T0665 )
- Lithium Chloride (Millipore Sigma, catalog number: L9659 )
- 7-Aminoactinomycin D (7-AAD; Millipore Sigma, catalog number: A9400 )
- Human insulin (Millipore Sigma, catalog number: 19278 )
- Monothioglycerol (MTG) (Millipore Sigma, catalog number: S5261 )
- HEPES (Millipore Sigma, catalog number: H4034 )
- Dextrose (Millipore Sigma, catalog number: G8270 )
- 2-mercaptoethanol (Millipore Sigma, catalog number: M7522 )
- Gelatin from porcine skin, Type A (Millipore Sigma, catalog number: G-1890 )
- Hexadimethrine bromide, Polybrene (Millipore Sigma, catalog number: H9268 )
- Fetal bovine serum, defined (HyClone, catalog number: SH30070.03 )
- Trypsin 0.05%/EDTA 0.5 mM (HyClone, catalog number: SH30236.02 )
- Recombinant Human Flt3-Ligand (Peprotech, catalog number: 300-19 )
- Recombinant Human FGF basic (Peprotech, catalog number:100-18B)
- Recombinant Human Stem Cell Factor (SCF) (Peprotech, catalog number: 300-07 )
- Recombinant Human Activin A (ActA) (Peprotech, catalog number: 120-14E )
- Recombinant Human Interleukin 3 (IL-3) (Peprotech, catalog number: 200-03 )
- Recombinant Human Thrombopoietin (TPO) (Peprotech, catalog number: 300-18 )
- Recombinant Human Interleukin 6 (IL6) (Peprotech, catalog number: 200-06 )
- Recombinant Human Bone Morphogenic Protein 4 (BMP4) (Peprotech, catalog number: 120-05ET )
- Recombinant Human Vascular Endothelial Growth Factor (VEGF) (Peprotech, catalog number: 100-20 )
- Recombinant Human Interleukin 7 (IL7) (Peprotech, catalog number: 200-07 )
- GSK-3β inhibitor (CHIR99021) (Tocris, catalog number: 4423 )
- Rock inhibitor (Tocris, catalog number: 1254 )
- Human ES cell qualified Matrigel (Corning, catalog number: 354277 )
- Calcium Chloride (CaCl2) (Alfa Aesar, catalog number: 33296 )
- Potassium Chloride (KCl) (Fisher ScientificTM, catalog number: BP366-1 )
- Sodium Chloride (NaCl) (Fisher ScientificTM, catalog number: S642-212 )
- Sodium Bicarbonate (Fisher ScientificTM, catalog number: S233-500 )
- Sodium azide, NaN3 (Fisher ScientificTM, catalog number: BP922-500 )
- BSA fraction V (Fisher ScientificTM, catalog number: BP1600-100 )
- Sodium Phosphate Dibasic Anhydrous (Na2HPO4) (Fisher ScientificTM, catalog number: BP332-1 )
- Polyvinyl alcohol (PVA) (MP Biomedicals, catalog number: 151-941-83 )
- Doxycycline (DOX) (MP Biomedicals, catalog number: 198955 )
- Antibodies (Table 1)
Table 1. List of antibodies used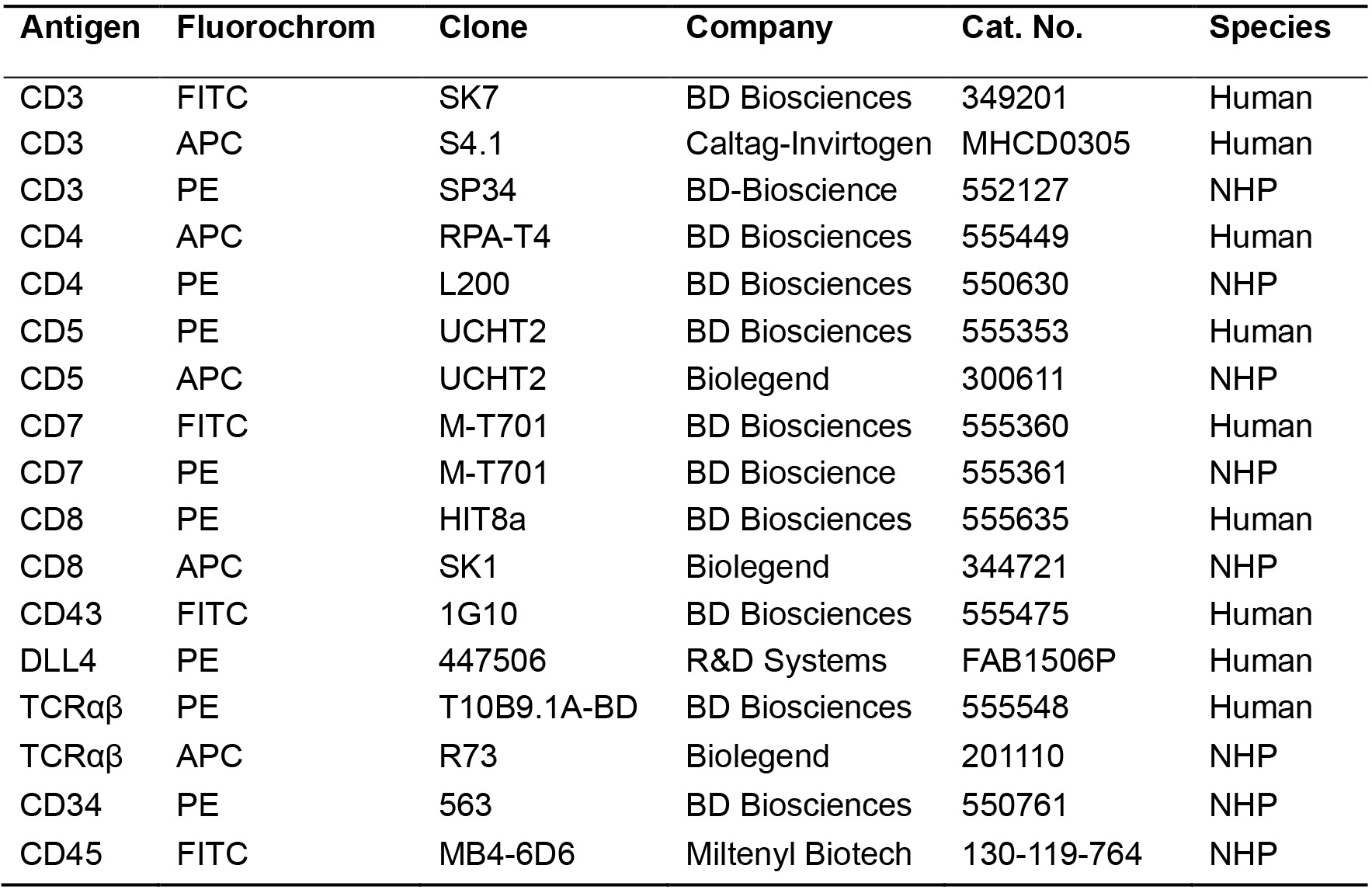
- Materials
- Cell strainer, 40 µm (Fisher ScientificTM, catalog number: 223663547 )
- Cell strainer, 70 µm (Fisher ScientificTM, catalog number: 22363548 )
- 9″ Pasteur pipets, Flint glass (Fisher ScientificTM, catalog number: 1367820D )
- Serological Pipettes (10 ml, VWR International, catalog number: 89130-898 )
- Serological Pipettes (95 ml, VWR International, catalog number: 89130-896 )
- Nalgene Disposable bottle top filter, Polyethersulfone membrane with 0.2 µm pore size (Fisher ScientificTM, catalog number: 595-4520 )
- 0.5 ml microcentrifuge tube, autoclavable (Fisher ScientificTM, catalog number: 05-408-120 )
- Serological pipet, 1 ml Nonpyrogenic (Fisher ScientificTM, catalog number: 13-678-11B )
- Borosilicate glass pipets, 5 ml (Fisher ScientificTM, catalog number: 1367827F )
- 5 ml Polystyrene round-bottom tube, 12 × 75mm, non-sterile (BD Bioscience, catalog number: 352008 )
- MACS separation columns, LS (Miltenyi Biotec, catalog number: 130-042-401 )
- MACS Multistand (Miltenyi Biotec, catalog number: 130-042-303 )
- Pre-separation filters with 30 µm nylon mesh (Miltenyi Biotec, catalog number: 130-041-407 )
- Tissue culture dishes, polystyrene 100 × 20 mm (Thermo Scientific, catalog number: 130182 )
- Tissue culture 6-well plate, Polystyrene flat bottom (Thermo Scientific, catalog number: 130184 )
- 15 ml Polypropylene Conical tubes (Thermo Scientific Nunc, catalog number: 339650 )
- 50 ml Polypropylene Conical tubes (Thermo Scientific, catalog number: 339652 )
- Steriflip Filter Units, 50 ml Vacuum filtration system with 0.22 µm pore size membrane (Millipore Sigma, catalog number: SCGP00525 )
- Serological pipet, 5 ml Nonpyrogenic (VWR, catalog number: 89130-896 )
- Serological pipet, 10 ml Nonpyrogenic (VWR, catalog number: 89130-898 )
- Sterling Nitrile-xtra powder-free exam gloves (Kimberly-Clark, catalog number: 53139 )
- Medium and solutions
- Human PSC growth Medium (see Recipes)
- MEF growth medium (see Recipes)
- NHP-iPSC Primate PSCs Medium (see Recipes)
- Mouse OP9/OP9-DLL4 bone marrow stromal cell culture medium (see Recipes)
- Human hematopoietic differentiation (OP9 coculture system) medium (see Recipes)
- NHP differentiation medium (see Recipes)
- IF4S Stock Medium (see Recipes)
- 5x PVA Stock Solution (see Recipes)
- IF9S Medium (see Recipes)
- T lymphoid differentiation medium (see Recipes)
- HBS saline solution (2x) (see Recipes)
- CaCl2 solution (2 M) (see Recipes)
- Gelatin solution (0.1% (wt/vol) (see Recipes)
- Magnetic cells sorting (MACS) buffer (see Recipes)
- Flow cytometry buffer (see Recipes)
- Reconstitution of cytokines (see Recipes)
- Collagenase solution (1 mg/ml) (see Recipes)
- Doxycycline (1 mg/ml) (see Recipes)
Equipment
- MACSQuant analyzer (Miltenyl Biotech, catalog number: 130-096-343 )
- CellometerR Auto 2000 cell Viability Counter (Nexcelom Bioscience, model: 2000 )
- Hemocytometer, Reichert Bright-Line counting chamber (Fisher ScientificTM, catalog number: 02-671-5 )
- MACSmix Tube Rotator (Miltenyi Biotec, catalog number: 130-090-753 )
- Water bath (Fisher Scientific, catalog number: 16-462-10 )
- Thermo IEC Centra CL2 Centrifuge (Thermo Scientific, model: 4992 )
- Microcentrifuge (Eppendorf, model: 5418 )
- Centricon Plus-70 Centrifugal Filter (Millipore Sigma, catalog number: UFC701008 )
- Sterile biosafety cabinet (The Baker Company, model: SG603 )
- 37 °C/5% CO2 incubator (Thermo Scientific, model: MCO-19A1 )
- Hypoxia incubator (Thermo Scientific, model: MCO-19M-PA )
- Inverted microscope with objective lenses 4x, 10x and 20x (Olympus, model: IX71 )
- Object marker, Cell dotter for inverted microscope (Nikon, catalog number: MBW10020 )
- Balance (Denver Instrument, model: APX60 )
- Milli-Q water purification system (Millipore, Billerica, MA, USA)
- Pipet-Aid, Filler/Dispensers (Drummond, model: 4-000-300 )
- Liquid waste disposal system for aspiration
- Beckman Optima XL-A analytical ultracentrifuge (Beckman Coulter, catalog number: 369005 )
- Bright-Field microscope (Olympus, model: CKX31SF )
Software
- FlowJoTM software (v10.6.1) (BD Bioscience, https://www.flowjo.com/)
- GraphPad Prism 7 (GraphPad, www.graphpad.com)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Kumar, A., D’Souza, S. S., Uenishi, G., Park, M. A., Lee, J. H. and Slukvin, I. I. (2020). Generation of T cells from Human and Nonhuman Primate Pluripotent Stem Cells. Bio-protocol 10(13): e3675. DOI: 10.21769/BioProtoc.3675.
分类
免疫学 > 免疫细胞分离 > 淋巴细胞
干细胞 > 多能干细胞 > 再生医学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link