- EN - English
- CN - 中文
Co-immunostaining of ICAM-1, ICAM-2, and CD31 in Mouse Kidney Glomeruli
小鼠肾小球ICAM-1、ICAM-2和CD31的联合免疫染色
发布: 2020年07月05日第10卷第13期 DOI: 10.21769/BioProtoc.3663 浏览次数: 4949
评审: Xiaoyi ZhengYing ShiAnonymous reviewer(s)

相关实验方案

研究免疫调控血管功能的新实验方法:小鼠主动脉与T淋巴细胞或巨噬细胞的共培养
Taylor C. Kress [...] Eric J. Belin de Chantemèle
2025年09月05日 3543 阅读
Abstract
Glomerulonephritis (GN) is a common pathological condition in chronic kidney diseases that often leads to end stage renal failure. Mac-1 (CD11b/CD18)-mediated neutrophil, macrophage, and dendritic cell glomerular infiltration leading to cellular dysfunction and destruction is an important disease mechanism. The cellular distribution and dynamics of the expression of Mac-1 ligands ICAM-1 and ICAM-2 in GN have not been well studied because of the difficulties in tissue staining and colocalizing glomerular cells with surface antigens. To improve the visualization of cell surface marker and antigen expression in kidney compartments, we have devised an even but mild fixation procedure employing p-formaldehyde-lysine-periodate (PLP) perfusion. A large panel of antibodies (Ab) against cell surface markers was used to identify kidney cell types and adhesion molecules. When confocal microscopy was used in visualizing glomerular adhesion molecule staining, the endothelial cells were found to specifically express CD31, and these cells express ICAM-2 constitutively. Though ICAM-1 was not expressed by glomerular endothelial cells in homeostasis, it was highly upregulated in mice with chronic GN and severe proteinuria. VCAM-1, a ligand for VLA-4 important in leukocyte migration, was not expressed in the glomerulus. The results highlight the importance of ICAM-1 in the infiltration of macrophages and dendritic cells in cGN. This report will provide a widely applicable procedure for yielding high quality confocal images and for the identification and quantitation of receptors and other cellular antigens expressed in different kidney compartments and cell types.
Keywords: Glomerulonephritis (肾小球肾炎)Background
The glomerulus plays a central role in the ultrafiltration function of the kidney by the formation of a semipermeable filtration barrier between the circulation and the urinary space (Dickinson, 2016). It is composed of a network of capillaries formed by fenestrated endothelial cells lined by podocytes with interdigitating foot-processes forming slit diaphragms for selective filtration and mesangial cells which provide mechanical support and contractile functions. Most chronic kidney diseases and a large percentage of end stage renal disease exhibit glomerulus involvement and glomerulonephritis (GN) is the underlying cause in 40% of patients with chronic kidney disease (Jha et al., 2013; Foster, 2016; Lindenmeyer and Kretzler, 2017). Leukocyte infiltration and immune complex deposition are largely responsible for the initiation and progression of GN with polymorphonuclear neutrophils (PMN) and macrophages constituting the predominant infiltrating cell types (Devi et al., 2013; Dickinson, 2016; Sung et al., 2017; Almarza Novoa et al., 2018). Leukocyte transmigration into the glomerulus is dependent on chemokine and adhesion molecule functions (Filippi, 2016; Sung et al., 2017). For PMN and monocyte adhesion and diapedesis into the subendothelial space and the mesangium, CD11b function is of particular significance (Ernandez and Mayadas, 2016; Filippi, 2016; Sung et al., 2017). This β2 integrin binds ICAM-1 and ICAM-2 which are expressed by glomerular intrinsic cells, especially the endothelial cells (Devi et al., 2013; Filippi, 2016; Sung et al., 2017). The expression of integrin β1 and β2 ligands including ICAM-1, ICAM-2, and VCAM-1 on endothelial cells are modulated by changes in the inflammatory environments but their expression within the glomerulus is not well-studied. To understand cellular interactions in GN pathogenesis, microscopic examination of the dynamics of cell type-specific expression of adhesion molecules is critical (Devi et al., 2013; Sung et al., 2017). Studies by confocal microscopy have provided a clearer understanding of leukocyte emigration in the glomerulus. Unequivocal definition of expression specificity in this microscopic technique requires (1) availability of glomerular cell type-specific Ab; (2) optimized methods for tissue preparation and fixation; (3) availability of Ab for detecting the antigenic epitopes; and (4) adequate signal to noise ratio. In the past, difficulties arising from one or more of these issues have resulted in suboptimal detection of integrin expression by glomerular cells. Some of the issues in kidney glomerulus staining are summarized below. The first is the high autofluorescence and nonspecific binding of renal tubules. The autofluorescence of cortical tubules is particularly notable. The problem of nonspecific staining is especially severe when secondary Ab are used. Availability of reagents poses another hurdle since fluorescence-tagged Ab for staining kidney glomerular intrinsic cell types such as podocyte and mesangial cells are not commercially available. Furthermore, with the staining of frozen sections, as is often the case, the images are diffuse and lack resolution compared to fixed tissues (Figure 1, cf. HLA-DR staining (arrows) in panel A–frozen and panel B–fixed sections). This report describes a mild kidney fixation method for preserving tissue architecture and Ab-binding antigenic epitopes. Ab conjugated with fluorescence tags for background reduction were used to identify glomerular cell expression of integrins in homeostatic and inflamed kidneys. The procedure yielded sharp images of glomerular endothelial cells expressing ICAM-1 and ICAM-2 and extraglomerular tubular cells expressing VCAM-1. The method is applicable to studies on cellular interactions and receptor and cytokine expression by other kidney cell types such as inflammatory cells, fibroblasts, and epithelial cells.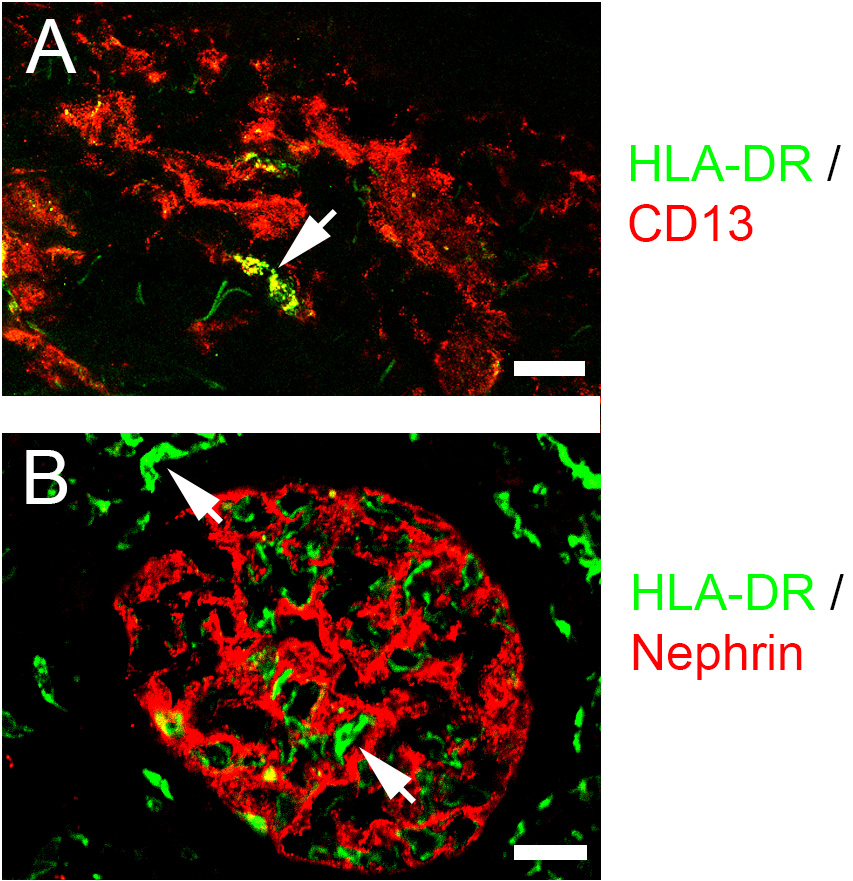
Figure 1. Comparison of immuno-fluorescence staining in frozen and fixed tissues. A. Human skin tissue was frozen in OCT, sectioned in 5 μm slices, fixed for 10 min in acetone at -20 °C, and stained with the indicated Ab as described in Procedure B. B. Human kidney tissue was fixed for 3 h in 2% phosphate-lysine-periodate (PLP), equilibrated in sucrose and OCT as described in Procedure A, sectioned, and stained with the indicated Ab as described in Procedure B. Confocal images were captured as described in Procedure C. Arrows indicate HLA-DR-stained cells. Scale bars = 25 μm.
Materials and Reagents
- 24-well plates (Sarstedt, catalog number: 83.1836 )
- Fisher Superfrost Plus Microscope Slide (Fisher Scientific, catalog number: 12-550-15 )
- Sharpie ultra fine point marker (Amazon.com, catalog number: 37001 )
- Gold Seal #3317 22 × 50 mm #1 cover glass (VWR, catalog number: 48404-053 )
- Tissue Cassettes (Fisher Scientific, catalog number: 15-200-403E )
- Base Molds, 15 × 15 mm, disposable (Fisher Scientific, catalog number: 22-363-553 )
- Pasteur pipet (Fisher Scientific, catalog number: 13-678-20D )
- Diamond glass cutter (Diamond knife) (EMS, catalog number: EMS 70038 )
- Coplin staining jar (Thermo, catalog number: E94 )
- BioAssay dish, square, 245 mm (Corning, catalog number: 431111 )
- Whatman grade 1 circular filter paper, 18.5 cm diameter (GE Healthcare, catalog number: 1001185 )
- 0.2 µm filter (Thermo, catalog number: 565-0020 )
- Whatman 3 MM Chromatography paper, 25 cm square (Fisher, catalog number: 05-716-6B )
- Microscope slide box (EMS, catalog number: 71470-W )
- NZM2328 mice (Originally from Jackson Laboratories; currently maintained in-house)
- O.C.T. Compound (Tissue-Tek OCT) (Sakura, catalog number: 4583 )
- Everbrite Hardset Mounting Medium without DAPI (Biotium, catalog number: 23003 )
- Polyclonal Ab, Goat Ab:
- Monoclonal anti-mouse Ab:
- Rat mAb: Alexa Fluor (A)-488-anti-mouse ICAM-2 [CD102, clone 3C4 (MIC2/4)] (Biolegend, catalog number: 105609 )
- Rat mAb: A-647-anti-mouse VCAM-1 (CD106, clone 429) (Biolegend, catalog number: 105712 )
- Hamster mAb: BV421 anti-mouse ICAM-1 (CD54 clone 3E2) (BD, catalog number: 564704 )
- Rat mAb: anti-mouse CD16/CD32 (clone 2.4G2) (Bio-X-Cell, catalog number: BE-0307 )
- Anti-human Ab
- mAb labeling kits for labeling unconjugated Ab:
- Alexa FluorTM 488 Antibody Labeling Kit (Thermo Fisher, Invitrogen, catalog number: A20181 )
- Alexa FluorTM 555 Antibody Labeling Kit (Thermo Fisher, Invitrogen, catalog number: A20187 )
- Alexa FluorTM 647 Antibody Labeling Kit (Thermo Fisher, Invitrogen, catalog number: A20186 )
- Pacific BlueTM Antibody Labeling Kit (Thermo Fisher, PacBlue, catalog number: P30013 )
- Phosphate-buffered formalin (10%; Fisher Scientific, catalog number: 22-110-869 )
- DAPI (Sigma, catalog number: 10236276001 )
- HCl (MP Biomedical, catalog number: 194697 )
- Triton X-100 (Sigma, catalog number: X100-500ML )
- Horse serum (Gibco, catalog number: 16050 )
- Chicken serum (Gibco, catalog number 16110-082)
- HEPES (free acid) (ICN, catalog number: 101926 )
- Dulbecco’s modified Eagle’s medium (DMEM; Gibco, catalog number: 11965-092 )
- Sucrose (Sigma, catalog number: S0389 )
- Ketamine (Ketaset; Zoetis, catalog number: EA2489-564 , 100 mg/ml)
- Xylazine (AnaSed; Akorn, catalog number: 59399-110-20 , NADA #139-236, 20 mg/ml)
- Heparin (Elkins-Sinn, catalog number: 6505-00-153-9740 , 1,000 units/ml)
- Monobasic sodium phosphate (NaH2PO4·H2O) (Baker, catalog number: 3818-5)
- Dibasic sodium phosphate (Na2HPO4·7H2O) (USB, catalog number: 20232 )
- NaCl (Fisher, catalog number: S271-1 )
- KH2PO4 (Baker, catalog number: 3246-5)
- KCl (Baker, catalog number: 3040-05)
- EDTA·H2O (Baker, catalog number: 4040-04)
- Lysine HCl (MP Biomedical, catalog number: 194697 )
- p-formaldehyde (Fisher, catalog number: 04042-500 )
- Sodium m-periodate (Sigma, catalog number: S-1147 )
- Dulbecco’s phosphate-buffered without Ca2+ and Mg2+ (PBS): Dilute 10× PBS (see Recipes) with double distilled (dd) H2O
- Sodium phosphate buffer, 1 M, pH 7.4 (1 M phosphate buffer) (see Recipes)
- Sodium phosphate buffer, 0.375 M, pH 7.4 (see Recipes)
- 10× PBS (see Recipes)
- NaOH, 10 N (see Recipes)
- HEPES, 1 M, pH 7.4 (see Recipes)
- EDTA, 0.5 M, pH 8.0 (see Recipes)
- 4% p-formaldehyde in 0.1 M phosphate buffer, pH 7.4 (see Recipes)
- Lysine phosphate (see Recipes)
- p-Formaldehyde-Lysine-sodium periodate (2% PLP) (see Recipes)
- Sucrose (30%) in 50 mM phosphate buffer, pH 7.4 (see Recipes)
- NaN3 (10%) (see Recipes)
- Detergent tissue extraction solution (see Recipes)
- Tissue blocking solution (see Recipes)
- Anesthesia mix (see Recipes)
- DAPI stock and working solutions (see Recipes)
Equipment
- 1 L beaker
- 1 L Erlenmeyer flas
- Stirring bar
- Fume hood
- Water bath
- Dissection tray (Carolina Biological Supply, catalog number: 629005 )
- Cryostat (Leica Biosystems, model: Leica CM1850 UV )
- Zeiss Laser Scanning Confocal Microscopy System with (Carl Zeiss, model: LSM 700 ):
- 405 nm, 488 nm, 555 nm, and 639 nm laser excitation lines
- EC Plan-Neofluar 10×/0.3. M27 objective
- Plan Apochromat 20×/0.8 M27 objective
- EC Plan-Neofluar 40×/1.30 Oil objective
- Plan-Apochromat 63×/1.4 Oil DIC M27 objective
- Revco Ultima II -70 °C Freezer (Thermo Scientific, model: ULT-2186-9-A )
- Constant pressure perfusion assembly with reservoirs in “Mariotte’s bottle” configuration:
A constant pressure reservoir assembly is constructed as shown in Figure 2A. It is consisted of 60 ml syringes with inlets made from #6 rubber stoppers and trimmed 5 ml pipets (Figure 2B). The syringe reservoirs are connected by 1/8” i.d. silicone tubings (Cole-Parmer, catalog number: EW-95886-03 ) through 1/16” i.d. Luer Fitting Adapters (Cole-Parmer, catalog number: GH41507-62 ) and regulated by a 3-way stopcock (Cole-Parmer, catalog number: 30600-08 ). The draining outlet is consisted of a 40” silicone tubing (3/32” i.d., Cole-Parmer, catalog number: EW-96201-84 ) with a 16 G needle (needle point trimmed) at one end to connect to the luer slip outlet of the 3-way valve and a luer lock adapter (Cole-Parmer, catalog number: GH41507-62 ) on the other end to connect with a 21 gauge butterfly vacutainer (BD, catalog number: 367296 ) for perfusion. The needle sheath of the vacutainer needle is trimmed to expose the needle head and fitted on the needle to stop excessive penetration (Figure 2C). The hydrostatic pressure is set at 32” H2O.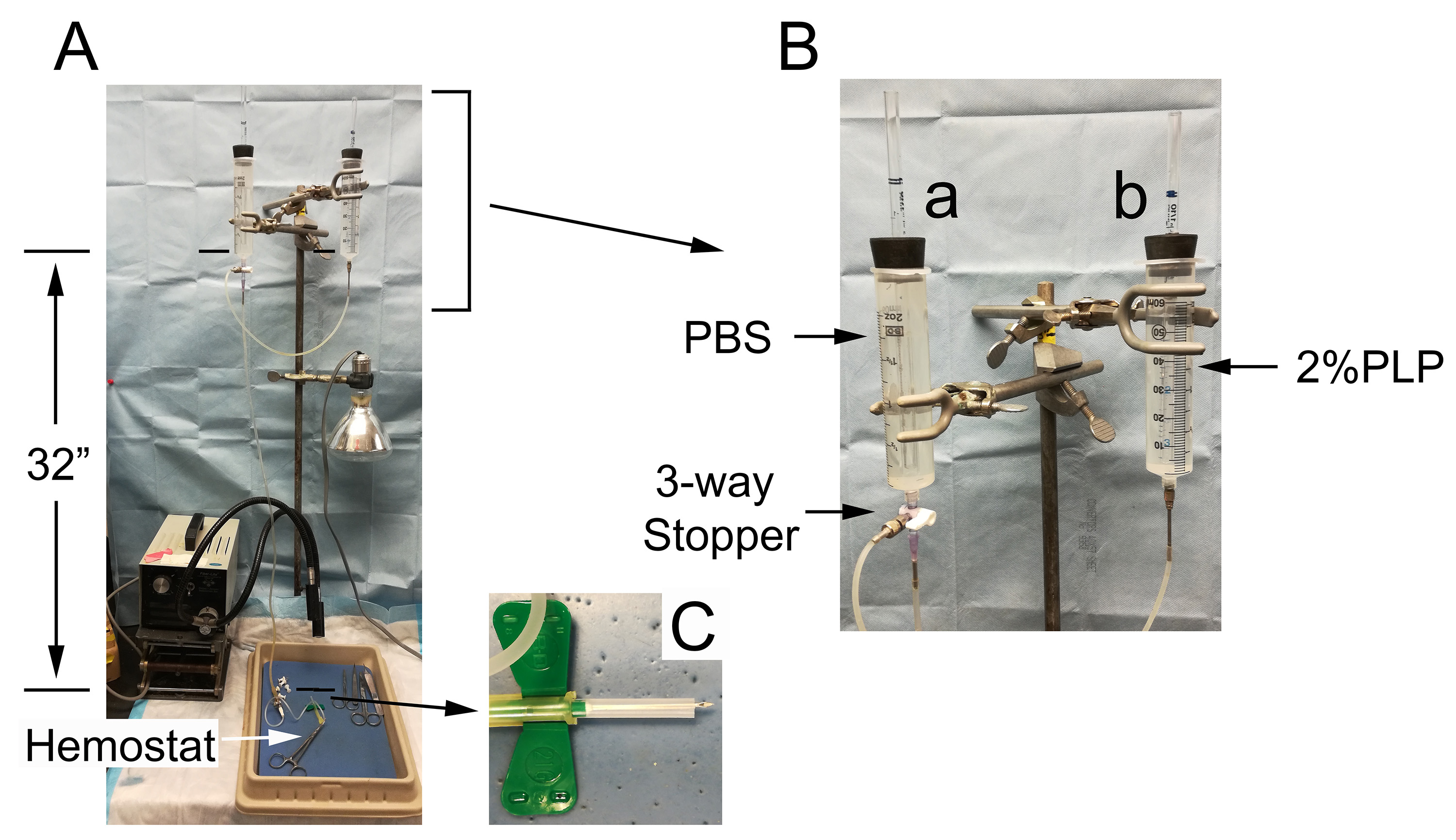
Figure 2. Mouse perfusion assembly. The perfusion assembly (A) is consisted of 2 connected 60 ml syringes in a Mariotte’s bottle configuration. Syringe a in (B) is fitted with a 3-way valve to regulate flow. The draining tubing outlet is connected with a 21 G vacutainer. The needle sheath is cut to size to expose the needle point (C) and left in place to prevent over-penetration of the heart. Perfusion through the vacutainer is stopped by clamping with a hemostat.
Software
- ZEN 2.3 SP1 FP3 (Black) 64 bit program for data acquisition (Zeiss, available here)
- ZEN Lite for data analysis (Zeiss, available here)
- Adobe Photoshop Version 20.0.4 20190227.r.76 (available here)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Sung, S. J. (2020). Co-immunostaining of ICAM-1, ICAM-2, and CD31 in Mouse Kidney Glomeruli. Bio-protocol 10(13): e3663. DOI: 10.21769/BioProtoc.3663.
分类
免疫学 > 免疫细胞功能 > 巨噬细胞
细胞生物学 > 细胞成像 > 共聚焦显微镜
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link