- EN - English
- CN - 中文
Live Cell Measurement of the Intracellular pH of Yeast by Flow Cytometry Using a Genetically-Encoded Fluorescent Reporter
一种基于流式细胞术的利用基因编码荧光报告测定酵母活细胞内pH值的方法
发布: 2020年06月20日第10卷第12期 DOI: 10.21769/BioProtoc.3653 浏览次数: 4822
评审: Steven BoeynaemsIndranil MalikAnonymous reviewer(s)
Abstract
The intracellular pH of yeast is a tightly regulated physiological cue that changes in response to growth state and environmental conditions. Fluorescent reporters, which have altered fluorescence in response to local pH changes, can be used to measure intracellular pH. While microscopy is often used to make such measurements, it is relatively low-throughput such that collecting enough data to fully characterize populations of cells is challenging. Flow cytometry avoids this drawback, and is a powerful tool that allows for rapid, high-throughput measurement of fluorescent readouts in individual cells. When combined with pH-sensitive fluorescent reporters, it can be used to characterize the intracellular pH of large populations of cells at the single-cell level. We adapted microscopy and flow-cytometry based methods to measure the intracellular pH of yeast. Cells can be grown under near-native conditions up until the point of measurement, and the protocol can be adapted to single-point or dynamic (time-resolved) measurements during changing environmental conditions.
Keywords: Flow cytometry (流式细胞术)Background
The intracellular pH of yeast is correlated with characteristics like viability and growth rate, and the regulation of intracellular pH consumes a large proportion of cellular energetic resources (Orij et al., 2011). However, intracellular pH can change rapidly and is highly environmentally sensitive, so it is crucial to have a fast, minimally perturbative method of measurement for this important aspect of cell physiology.
Genetically-encoded biosensors that convert local concentrations of a compound of interest into fluorescent readouts have revolutionized our ability to characterize the intracellular environment. For some sensors, the absolute fluorescence intensity is correlated with the readout. This can be a problem when performing in-cell measurements, since the fluorescence depends both on the sensor expression level, which varies cell to cell, and the characteristic of interest. Ratiometric sensors, which depend on the ratio of fluorescence in two different parts of the spectrum in the same fluorophore, do not suffer from this drawback. One such sensor, pHluorin (Miesenböck et al., 1998), is a pH-sensitive fluorescent biosensor based on GFP that can be used to measure intracellular pH; the emission intensity (measured around 520 nm) after excitation in the near-UV and blue (405 and 488 nm typically) varies with pH such that the ratio of emission intensities can be related to pH.
In this protocol, we outline how to use pHluorin to measure intracellular pH in living budding yeast cells using flow cytometry. Flow cytometry is a method that combines microfluidic focusing and optical interrogation to measure the fluorescence of single cells in liquid culture with little to no special sample preparation required. Others have also used this method to measure intracellular pH in yeast cells (Weigert et al., 2009; Valkonen et al., 2013). The advantage of flow cytometry is that it is much higher throughput than microscopy-based methods (Bagar et al., 2009; Orij et al., 2009), while still being able to characterize individual cells and measure the fluorescence of multiple fluorophores. This access to both single-cell measurements and enough data to generate population-level statistics with a great deal of confidence is highly valuable. One potential downside to using flow cytometry to analyze the fluorescence of biosensors is that the spatial distribution of the fluorophore within the cell is not accessible (at least with traditional flow cytometry), as only a single, average fluorescence value in each channel is reported for each event (cell). However, for intracellular pH measurements in particular, the variation in any compartment (here, the cytosol, although pHluorin can be targeted to other organelles) (Orij et al., 2009) is expected to be minimal due to the unique properties of proton exchange in buffered aqueous solutions such as the cellular interior (Boron, 2004).
The outline of a typical experiment is illustrated in Figure 1. Cells expressing pHluorin are suspended in buffer of known pH and an ionophore, in this case nigericin, is added. This addition makes the cell membrane permeable to protons and thus equilibrates the intracellular and extracellular pH. The ratiometric fluorescence of pHluorin is measured for these cells with known intracellular pH, and then these data are used to construct a calibration curve that can be used to convert measured fluorescence ratios in other cells to real intracellular pH values.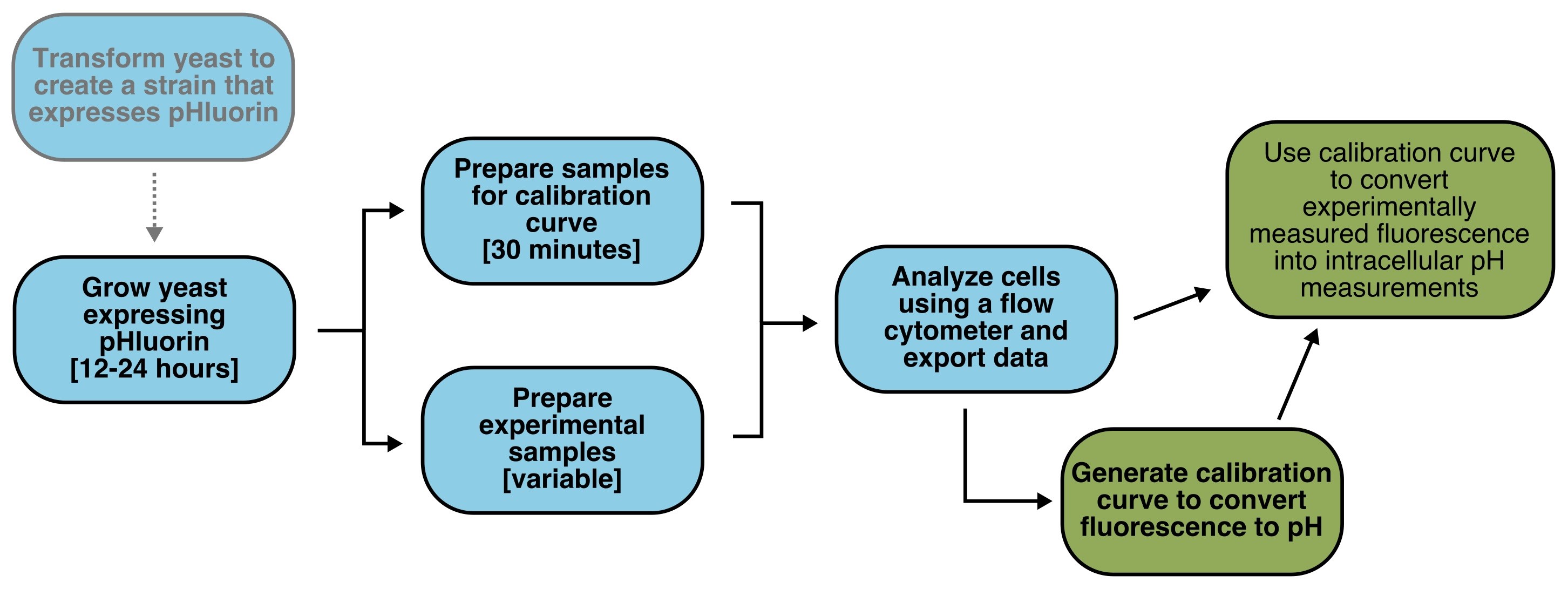
Figure 1. Flowchart of Protocol. Experimental steps are colored blue, analysis steps are in green. Optional steps are in gray text.
Materials and Reagents
- 50 ml conical flasks (Olympus Plastics, catalog number: 28-108 )
- 0.22 μm filter (Fisher, catalog number: CLS 431118 )
- 5 ml polystyrene round-bottom tubes, 12 x 75 mm style (Corning Falcon, catalog number: 358058 )
- Strain of Saccharomyces cerevisiae from the S288C background, such as BY4741/2/3
Alternatively, another strain of yeast may be used, as long as both non-fluorescent and pHluorin-expressing strains are available or can be made by the investigator (see optional reagents below). More information on yeast strains and genotypes can be found at https://wiki.yeastgenome.org/index.php/Commonly_used_strains. - 2-Deoxy-D-glucose (Sigma, Sigma Life Sciences, catalog number: D8375-5G )
- Nigericin (Adipogen, Adipogen Life Sciences, catalog number: AG-CN2-0020 ), prepared as a 10 mM stock in 100% ethanol and stored at -20 °C
- Yeast Nitrogen Base (YNB) + Nitrogen (Sunrise Scientific, catalog number: 1501-250 )
- Synthetic Complete (SC) dropout mix (Sunrise Scientific, catalog number: 1300-030 )
- D-(+)-Glucose (Research Products International, catalog number: G32040-5000.0 )
- MES (Fisher, catalog number: BP300-100 )
- HEPES (GoldBio, catalog number: H-400-1 )
- KCl (potassium chloride) (Fisher, catalog number: P217-3 )
- NaCl (sodium chloride) (Fisher, catalog number: BP358-212 )
- 2 M HCl (hydrochloric acid) (Fisher, catalog number: A144-212 )
- 2 M KOH (potassium hydroxide) (Sigma, catalog number: 484016-1KG )
- Ammonium acetate (Sigma, catalog number A1542-250G)
- 2X Calibration Curve Buffer (see Recipes)
- Yeast growth media (see Recipes)
Optional reagents
- pCGT05 or pHluorin expression vector (see Notes and attached plasmid map for more details; if the strain expressing pHluorin does not already exist and must be made by the investigator, then this reagent is not optional)
- PmeI (New England Biolabs, catalog number: R0560S ; comes with CutSmart® Buffer)
- Salmon testes DNA (Millipore Sigma, catalog number: D1626-250MG ) prepared as a 2 mg/ml solution in TE Buffer (see Recipes)
- Molecular Biology grade agarose (Apex Bioresearch Products, catalog number: 20-102GP )
- 10x TBE Buffer (Bio-Rad, catalog number: 161-0733 ), diluted to 1x in ultrapure water
- SCD (2%) plates without leucine, for selection
- Tris-HCl (Fisher, catalog number: BP153-1 )
- Tetrasodium EDTA (Fisher, catalog number: S311-100 ) prepared as a 1 M pH 8.0 stock
- Lithium Acetate (Sigma, catalog number: L6883-1KG )
- Polyethylene glycol (PEG) 3350 (Sigma, catalog number: P4338-500G )
Equipment
- Microcentrifuge capable of spinning 1.5 ml tubes at 3,000 x g
- BD Biosciences LSR Fortessa flow cytometer (see note for instrument settings), or any flow cytometer capable of exciting between 380 and 410 nm and between 470-490 nm, and measuring emission (for both excitations) between 500 and 550 nm (see Notes). Other laser configurations could also be used; see Miesenböck et al. 1998 for full characterization of pHluorin
- Electronic pH meter such as the Mettler Toledo SevenCompact pH meter S220 (Mettler Toledo, model: 30019032 )
- (Optional) For yeast transformation
- Heat block/dry bath/water bath
- Mold for casting agarose gels
- Standard electrophoresis power supply and setup for running agarose gels
- Heat block/dry bath/water bath
Software
- Software that can read FCS files. In this protocol we use R (CRAN, https://www.r-project.org/) with the flowCore package (Bioconductor, https://bioconductor.org/packages/release/bioc/html/flowCore.html); see example script for data analysis pipeline
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Triandafillou, C. G. and Drummond, D. A. (2020). Live Cell Measurement of the Intracellular pH of Yeast by Flow Cytometry Using a Genetically-Encoded Fluorescent Reporter. Bio-protocol 10(12): e3653. DOI: 10.21769/BioProtoc.3653.
分类
微生物学 > 微生物细胞生物学 > 基于细胞的分析方法 > 离子分析
细胞生物学 > 基于细胞的分析方法 > 流式细胞术
细胞生物学 > 细胞成像 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link