- EN - English
- CN - 中文
Preparation of a Bacteriophage T4-based Prokaryotic-eukaryotic Hybrid Viral Vector for Delivery of Large Cargos of Genes and Proteins into Human Cells
用于将基因和蛋白质转运至人体细胞中的基于噬菌体T4的大容量原核真核杂交病毒载体的制备
发布: 2020年04月05日第10卷第7期 DOI: 10.21769/BioProtoc.3573 浏览次数: 5632
评审: Miao HeVasudevan AchuthanRakesh Bam
Abstract
A viral vector that can safely and efficiently deliver large and diverse molecular cargos into cells is the holy grail of curing many human diseases. Adeno-associated virus (AAV) has been extensively used but has a very small capacity. The prokaryotic virus T4 has a large capacity but lacks natural mechanisms to enter mammalian cells. Here, we created a hybrid vector by combining T4 and AAV into one nanoparticle that possesses the advantages of both. The small 25 nm AAV particles are attached to the large 120 nm x 86 nm T4 head through avidin-biotin cross-bridges using the phage decoration proteins Soc (small outer capsid protein) and Hoc (highly antigenic outer capsid protein). AAV thus “piggy-backed” on T4 capsid, by virtue of its natural ability to enter many types of human cells efficiently acts as a “driver” to deliver large cargos associated with the T4 head. This unique T4-AAV hybrid vector approach could pave the way for the development of novel therapeutics in the future.
Keywords: Bacteriophage T4 (噬菌体T4)Background
There has been a desperate need for new and efficient delivery vehicles that can transport large cargos of genes and proteins into human cells to stimulate the production of therapeutic biomolecules and/or to repair cellular and genetic defects. Such vehicles will allow translation of the rapidly emerging technologies such as CRISPR, CAR T cells and so on, into therapies, both for mass applications as well as for personalized medicine (Stewart et al., 2016).
Assembling nanoparticles with different properties into a hybrid complex is a powerful strategy for developing novel functional materials, as these hybrid complexes exhibit collective and collaborative attributes, some of which might be different from those exhibited by the individual particles (Ghosh et al., 2012; Wang et al., 2014). Virus nanoparticles representing a fascinating class of protein-polynucleotide supramolecular structures are an ideal natural resource to construct such hybrids. Considering different shapes and sizes ranging from tens to hundreds of nanometers and their documented ability for intracellular delivery, combining different viral building blocks into a multifunctional nanoparticle will greatly accelerate future therapies.
Molecular and genetic analyses led to the engineering of an empty phage T4 shell (head or capsid) lacking the outer capsid proteins Soc (small outer capsid protein; 870 copies/head; 9 kDa) and Hoc (highly antigenic outer capsid protein; 155 copies/head; 41 kDa), neck, or tail as a delivery vehicle (Zhang et al., 2011). Both Soc and Hoc are non-essential for phage infection and exhibit high binding affinity and specificity to T4 capsid surface. Soc binds as a trimer at the quasi three-fold axes whereas Hoc binds as a monomer at the center of each gp23 capsomer (hexamer). Foreign proteins can be efficiently displayed on T4 head through fusion to N and C termini of Soc or the N terminus of Hoc which are well exposed (Tao et al., 2013).
The 25 nm adeno-associated virus (AAV) has been extensively used as a gene delivery vehicle but it has a very small capacity and cannot deliver proteins. The 120 x 86 nm prokaryotic virus, the bacteriophage T4, on the other hand has a large capacity but it lacks natural mechanisms to enter human cells. It has no tropism to human cells, has no known toxicity or pathogenicity, and exhibits no preexisting immunity. We reported the development of the phage-virus hybrid vector by integrating these two most well-characterized delivery vehicles into one (Figure 1) (Zhu et al., 2019). A specific biomolecular avidin-biotin interaction was selected as a driving force to mediate the interaction between the T4 head and AAV particle. It is also one of the strongest no-covalent interactions known in nature (Kd = 10-15 M), making it ideal as a molecular bridge between the two nanoparticles (Jain and Cheng, 2017). We engineered it in such a way that all the different compartments of the T4-AAV nanoparticle could be utilized for loading cargo molecules. These include the interior of the T4 capsid shell that can accommodate 170 kbp of DNA cargo (Zhang et al., 2011), the interiors of attached AAV shells each accommodating 5 kbp of DNA cargo (Muzyczka, 2014), and the exterior of the T4 shell that can be decorated with up to 1,025 molecules of protein cargo (Li et al., 2007; Shivachandra et al., 2007; Tao et al., 2013 and 2018). This system has been optimized such that varieties of customized cargos can be prepared by simple mixing of interacting components that have nanomolar or sub-nanomolar affinities and by using a powerful DNA packaging motor (Leffers and Rao, 1996; Fuller et al., 2007). Furthermore, different cargo molecules can be mixed and matched, quantified, and tuned as desired.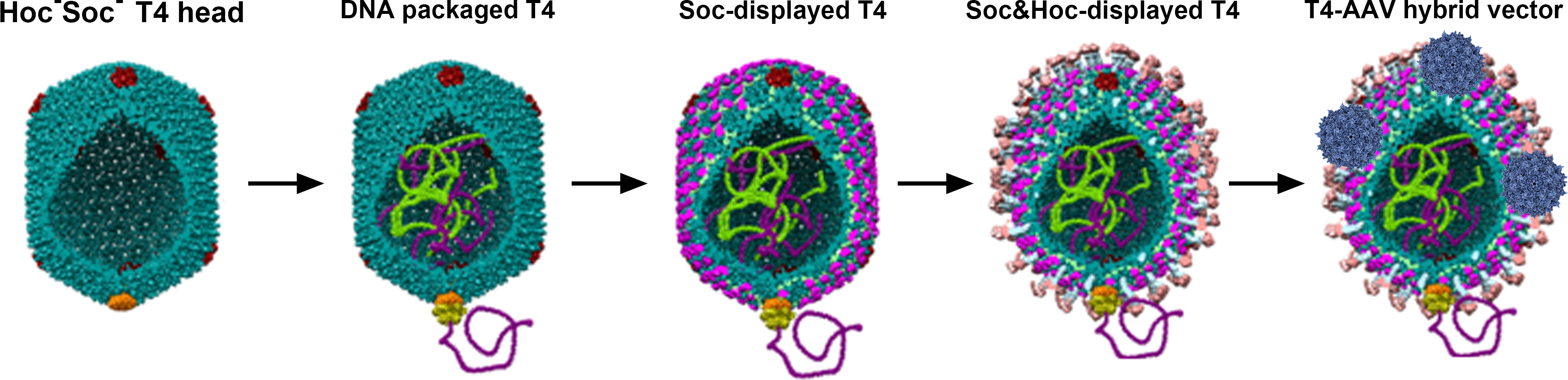
Figure 1. Schematic of DNA & protein carried T4 and T4-AAV hybrid vector
In this protocol, we describe the construction, expression, purification, and biotinylation of Soc and BAP-Hoc (BAP, Biotin Acceptor Peptide), and the assembly of Soc-biotin-avidin and Hoc-BAP-biotin-avidin. Soc and BAP-Hoc were biotinylated in vitro using biotin N-Hydroxysuccinimide (NHS) esters and BirA ligase, respectively. NHS-activated biotins react efficiently with primary amino groups (-NH2) of Soc protein (the side chain of lysine residues and the N-terminus) to form stable amide bonds. BirA biotin ligase site-specifically biotinylates a lysine side chain within a 15-amino acid BAP peptide fused to the N terminus of Hoc protein. We also provide a description of the production, purification, and modification of AAV and T4 head. Finally, we describe the methodology for generating the hybrid T4-AAV particle using biotinylated AAV, T4 head, and the “linker” molecules, Soc-biotin-avidin and Hoc-BAP-biotin-avidin (Figure 2). This protocol describes the assembly of hybrid T4-AAV vector in ~2 weeks. Although this study focused on T4 and AAV to conceptually demonstrate the utility of combining distinct viral building blocks, in a broader context, other hybrid vectors containing the T4 ‘carrier’ complexed with eukaryotic viruses, bacteria, or polymers using bioaffinity attachment technology can be envisioned for delivery of multivalent gene and protein therapeutics (Akin et al., 2007; Yoo et al., 2011).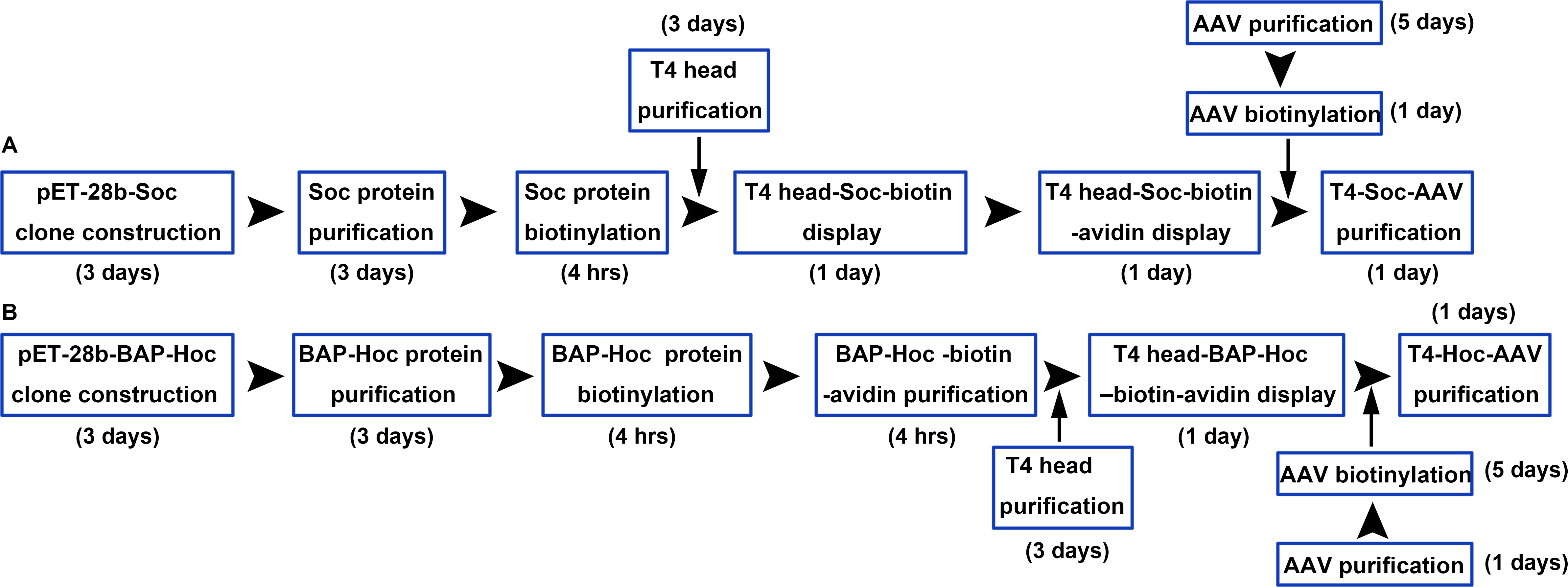
Figure 2. Workflow for construction of T4-Soc-AAV (A) and T4-Hoc-AAV (B) vectors. The T4 capsid lattice is decorated with two nonessential outer capsid proteins, Soc and Hoc. Soc and BAP-Hoc proteins were expressed in E.coli, purified by chromatography, and biotinylated. Two bridge molecules were constructed to attach AAV to the T4 head, Soc-biotin-avidin and Hoc-biotin acceptor peptide (BAP)-biotin-avidin. Soc and Hoc attach to the T4 head, and avidin attaches to biotinylated AAV (bioAAV) on the other side. T4-Soc-AAV (A) and T4-Hoc-AAV (B) conjugates were then purified by Ni2+-NTA agarose chromatography.
Materials and Reagents
- 15 ml tubes (GeneMate, catalog number: C3394-1 )
- 500 ml flask (VWR, catalog number: 89095-276 )
- 50 ml tubes (CELLSTAR, catalog number: 227270 )
- 125 ml flask (VWR, catalog number: 89095-258 )
- 15 ml Corex glass tubes (Corning)
- 1.7 ml sterile Eppendorf tube (Eppendorf, catalog number: 022431021 )
- Millex-GV Syringe Filter Unit, 0.22 µm (Millipore-Sigma, catalog number: SLGV033RS )
- HisTrap HP histidine-tagged protein purification columns (GE Healthcare, catalog number: 17524701 )
- Hi-load 16/600 Superdex200pg Gel filtration chromatography column (GE Healthcare, catalog number: 28989335 )
- Zeba Spin Desalting Columns (Thermo Scientific, catalog number: 89893 )
- Amicon Ultra-4 Centrifugal Filter Units (Millipore-Sigma, catalog number: UFC810008 )
- Competent E. coli DH5α cells (New England Biolabs, catalog number: C2987 )
- Competent E. coli BL21-CodonPlus (DE3)-RIPL cells (Agilent Technologies, catalog number: 230280 )
- HEK293T cells (ATCC, catalog number: ACS-4500 )
- Plasmids
a.pET-21a-BirA (Addgene, catalog number: 20857 )
b.pAAV-DJ (Cell Biolabs, catalog number: VPK-420-DJ )
c.pAAV-Helper (Cell Biolabs, catalog number: VPK-400-DJ )
d.pAAV-GFP (Cell Biolabs, catalog number: AAV-400 )
e.pAAV-luciferase (Cell Biolabs, catalog number: AAV-321 )
f.pAAV-mCherry (Cell Biolabs, catalog number: VPK-410 ) - Luria-Bertani (LB) medium (Quality Biological, catalog number: 10128-254 )
- Kanamycin (Gold Biotechnology, catalog number: K-120 )
- 2x Phusion High-Fidelity PCR Master Mix (Thermo Scientific, catalog number: F531S )
- Restriction enzymes
a.FastDigest NheI (Thermo Scientific, catalog number: FD0973 )
b.FastDigest HindIII (Thermo Scientific, catalog number: FD0504 )
c.FastDigest BglII (Thermo Scientific, catalog number: FD0084 )
d.FastDigest XhoI (Thermo Scientific, catalog number: FD0694 ) - FastAP Thermo sensitive Alkaline Phosphatase (Thermo Scientific, catalog number: EF0654 )
- T4 DNA Ligase (Thermo Scientific, catalog number: EL0014 )
- AccuGENETM 10x TBE Buffer (Lonza, catalog number: 50843 )
- QIAquick Gel Extraction Kit (QIAGEN, catalog number: 28706 )
- QIAprep Spin Miniprep Kit (QIAGEN, catalog number: 27106 )
- CompactPrep Plasmid Midi Kit (QIAGEN, catalog number: 12843 )
- Chloramphenicol (Amresco, catalog number: 0230-100G )
- Complete proteinase inhibitor cocktail (Roche, catalog number: 4693159001 )
- Isopropyl β-D-1-thiogalactopyranoside (IPTG) (Gold Biotechnology, catalog number: 12481C )
- EDTA (Quality Biological, catalog number: 351-027-721 )
- Dimethyl sulfoxide (DMSO) (ATCC, catalog number: 80713185 )
- Sodium acetate (Sigma-Aldrich, catalog number: 229873 )
- Ethanol (VWR, catalog number: BDH 1156-4LP )
- Deoxyribonuclease I (DNase I) (Sigma-Aldrich, catalog number: D5025 )
- Benzonase endonuclease (Millipore-Sigma, catalog number: 1016540001 )
- M9CA medium powder (Amresco, catalog number: J864-500G )
- Proteinase K Solution (Thermo Scientific, catalog number: AM2546 )
- HiTrap DEAE Sepharose FF (GE Healthcare, catalog number: 17515401 )
- HiTrap AVB Sepharose HP (GE Healthcare, catalog number: 28411211 )
- Bovine Serum Albumin, Acetylated (Promega, catalog number: R3961 )
- 4-20% Tris-Glycine Mini Gels (Thermo Scientific, catalog number: XP04205BOX )
- Coomassie Brilliant Blue R-250 Staining Solution (BIO-RAD, catalog number: 1610436 )
- Chloroform, HPLC Grade (Thermo Scientific, catalog number: AA43685M1 )
- Slide-A-Lyzer Dialysis Cassettes (Thermo Scientific, catalog number: 66380 )
- PBS (10x), pH 7.4 (Quality Biological, catalog number: 50146771 )
- Lipofectamine 2000 (Thermo Scientific, catalog number: 11668027 )
- QuickTiter AAV Quantitation Kit (Cell Biolabs, catalog number: VPK-145 )
- EZ-Link NHS-Biotin Reagents (Thermo Scientific, catalog number: 20217 )
- Dulbecco's Modified Eagle Medium (DMEM) (Thermo Scientific, catalog number: 10313039 )
- Sodium Chloride, 5 M (Quality Biological, catalog number: 351036491 )
- Ni-NTA Agarose (QIAGEN, catalog number: 30210 )
- Biotin (Sigma-Aldrich, catalog number: B4639 )
- ATP (Sigma-Aldrich, catalog number: A1852-1VL )
- pET-21a-BirA (Addgene, catalog number: 20857 )
- Tryptone (GROWCELLS, catalog number: MCMA-0901 )
- Yeast extract (Affymetrix, catalog number: 8013-01-2 )
- NaCl (Affymetrix, catalog number: 21618-5KG )
- Dextrose (AMRESCO, catalog number: 0188-IKG )
- Na2HPO4 (VWR, catalog number: 0404-2.5KG )
- KH2PO4 (Alfa Aesar, catalog number: 11594 )
- Tris-HCl (Quality Biological, catalog number: 351-006-101 )
- Imidazole (Alfa Aesar, catalog number: 11594 )
- Methanol (VWR, catalog number: BDH 1135-4LP )
- Acetic acid (VWR, catalog number: BDH3908-3.8LP )
- MgSO4 (Sigma-Aldrich, catalog number: M7506 )
- Moore’s medium (see Recipes)
- 1 M IPTG (see Recipes)
- HisTrap binding buffer (see Recipes)
- HisTrap elution buffer (see Recipes)
- Gel filtration buffer (see Recipes)
- SDS-PAGE destaining solution (see Recipes)
- M9CA medium (see Recipes)
- PI-Mg buffer (see Recipes)
- Head binding buffer (see Recipes)
- Head elution buffer (see Recipes)
- DNA packaging buffer (see Recipes)
- PBS-MK buffer (see Recipes)
- DMEM cell culture complete medium (see Recipes)
Equipment
- Pipettes (VWR, catalog number: 89130898 )
- Water bath (VWR, model: 1217 )
- -80 °C freezer (Panasonic, model: MDF-U76VC )
- SS34 rotor (SORVALL)
- GS3 rotor (SORVALL)
- AM 2.18 rotor
- PCR machine (BIO-RAD, model: T100 thermal cycler )
- Sorvall RC-5C plus centrifuge (Thermo Scientific, model: SorvallTM RC-5C plus )
- French press (Thermo Scientific)
- AKTA-prime system (Amersham Biosciences)
- AKTA Fast Protein Liquid Chromatography (FPLC) system (GE Healthcare, model: UPC-900 )
- NanoDrop 2000 spectrophotometer (Thermo Scientific, model: NanoDropTM 2000 )
- Shaking incubator (New Brunswick Scientific, model: Innova 43 )
- ChemiDoc Imaging Systems (BIO-RAD, 731BR02947 )
- MilliQ water machine (Millipore-Sigma, lot number: F8BA18025 )
- Bench centrifuge (Eppendorf, model: 5415D )
- Autoclave (Tuttnauer, model: 3870EA-B/L )
- High-speed multi-function centrifuge (Jouan, model: MR-23i )
Software
- ImageJ (National Institutes of Health Image, https://imagej.nih.gov/ij/)
- Quantity One software (Bio-Rad, CA)
- SnapGene (GSL Biotech LLC)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Zhu, J., Tao, P., Mahalingam, M. and Rao, V. (2020). Preparation of a Bacteriophage T4-based Prokaryotic-eukaryotic Hybrid Viral Vector for Delivery of Large Cargos of Genes and Proteins into Human Cells. Bio-protocol 10(7): e3573. DOI: 10.21769/BioProtoc.3573.
- Zhu, J., Tao, P., Mahalingam, M., Sha, J., Kilgore, P., Chopra, A. K. and Rao, V. (2019). A prokaryotic-eukaryotic hybrid viral vector for delivery of large cargos of genes and proteins into human cells. Sci Adv 5(8): eaax0064.
分类
分子生物学 > DNA > DNA 重组
分子生物学 > DNA > 基因表达
分子生物学 > 纳米颗粒 > 植物源纳米颗粒
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













