- EN - English
- CN - 中文
Determination of Flavin Potential in Proteins by Xanthine/Xanthine Oxidase Method
黄嘌呤/黄嘌呤氧化酶法测定蛋白质中的黄素电位
发布: 2020年04月05日第10卷第7期 DOI: 10.21769/BioProtoc.3571 浏览次数: 4981
评审: Manjula MummadisettiAmit DeyAnonymous reviewer(s)
Abstract
This protocol describes a simple xanthine/xanthine oxidase enzymatic equilibration method for determination of the redox potential of a flavin. As an example of the use of this method, we determine the reduction potential of the covalently bound FAD cofactor (Em = -55 mV) in the SdhA flavoprotein subunit of succinate dehydrogenase from Escherichia coli. In principle, this method can be used routinely to determine the redox potential of flavin cofactors in any simple flavoprotein from equilibrium concentrations with an appropriate reference dye of known Em without the use of sophisticated electrochemical equipment.
Keywords: Flavin (黄素)Background
Several biophysical methods can be used to measure the reduction midpoint potential (Em) of flavins in a protein. These potentiometric methods usually rely on the electrochemical coupling between the protein of interest and an electrode. For example, in complex flavoproteins harboring additional cofactors such as iron-sulfur centers and quinones, electrochemical methods and Electron Paramagnetic Resonance (EPR) spectroscopy are often used to determine the Em of the redox centers (Kowal et al., 1995; Saenger, et al., 2005; Hudson et al., 2005; Cheng et al., 2015). However, in simple flavoproteins that contain only flavin redox centers, the co-factor can be directly studied by conventional optical spectrophotometry which does not require special electrochemistry equipment or expensive EPR instrumentation. In 1990 Vincent Massey introduced a simple method that allows determination of the flavin reduction potential from the equilibrium concentrations of the oxidized and reduced partners, i.e., flavoprotein and a reference dye (Massey, 1991). The method does not directly measure the reduction potential of the flavin but rather determines a difference between Em values of the flavin and a reference dye. The scheme in Figure 1 describes the method. The xanthine/xanthine oxidase system provides a slow continuous reduction of the indicator dye and the flavin in the presence of benzyl viologen (BV) or methyl viologen (MV) which ensures the rapid equilibration of reducing equivalents. This allows slow changes in equilibrium of the reduced and oxidized forms of the protein and dye until both are completely reduced. A series of spectra recorded over the course of the reaction is used to calculate the ratio of the oxidized and reduced forms of flavin and dye. The Nernst plot of an equilibrium concentration, the dye against the flavoprotein allows determination of the shift in the Em of the protein in comparison with the dye. Because some of the components used in this method are low potential chemicals and proteins (viologen, reference dyes, and many flavoproteins) that are readily oxidized by even trace amounts of oxygen, a requirement for this system is that strict anaerobiosis be maintained.
Here we show an example of how this method is applied to determine the reduction potential of covalently bound FAD in the SdhA flavoprotein of succinate dehydrogenase. SdhA is the flavoprotein component of the four subunit membrane-bound succinate:ubiquinone reductase (i.e., complex II) which is part of the TCA (Krebs) cycle and electron transport chain of the mitochondrion and many bacteria. Complex II couples the reaction of succinate oxidation to fumarate with ubiquinone reduction to ubiquinol. The FAD co-factor is involved in the reversible succinate-fumarate conversion. The Em for free FAD in solution is -219 mV, whereas covalent attachment of FAD to SdhA considerably raises the potential of the flavin. In this example, we use the redox dye indigo-tetrasulphonate (ITS) (Em = -46 mV) and have determined that the EmFAD for the Escherichia coli SdhA protein is -55 mV. In principle, this simple method can be used for any flavoprotein by using a redox dye with a suitable reduction potential (i.e., a potential within ±30 mV to that expected for the flavoprotein being determined). This method has also been validated for the determination of the reduction potential of many other flavoproteins (see Christgen et al., 2019) and several heme proteins that suggests that the method could be widely useful for most heme proteins (Efimov et al., 2014).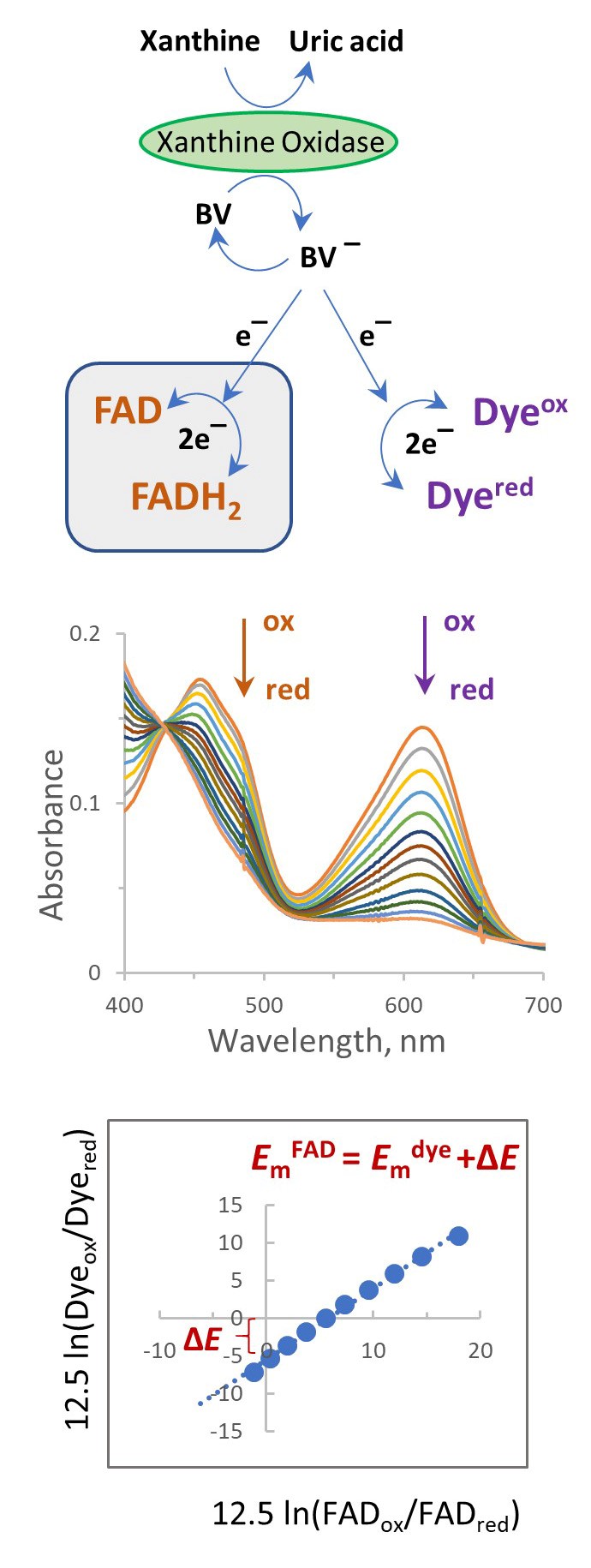
Figure 1. Spectrophotometric method for determination of the Em of a flavin using a reference dye in the presence of xanthine/xanthine oxidase. Most suitable dyes undergo 2-electron reduction (see Table 1).
Materials and Reagents
- 0.2 µm filter (Corning) (Sigma-Aldrich, catalog number: CLS431229 )
- Potassium phosphate dibasic, anhydrous (Sigma-Aldrich, catalog number: 795496 )
- Potassium phosphate monobasic, anhydrous (Sigma-Aldrich, catalog number: 795488 )
- Xanthine (Sigma-Aldrich, catalog number: X7375 )
- Xanthine oxidase, lyophilized powder (Sigma-Aldrich, catalog number: X4376 )
- E. coli SdhA
E. coli SdhA was isolated with an N-terminally fused His-tag as described in Maklashina et al., 2018. - Glucose (Sigma-Aldrich, catalog number: G8270 )
- Catalase (Sigma-Aldrich, catalog number: C30 )
- Glucose oxidase (Sigma-Aldrich, catalog number: G2133 )
- Potassium indigotetrasulfonate (ITS) (Sigma-Aldrich, catalog number: 340596 )
- Benzyl viologen (Sigma-Aldrich, catalog number: 271845 ) or Methyl Viologen (Sigma-Aldrich, catalog number: 856177 )
- Flavoprotein, isolated SdhA (see the expression and purification method; Maklashina et al., 2018)
- Ultra High Purity 5.0 Grade Argon (Airgas, catalog number: AR UHP300 ) or Ultra High Purity 5.0 Grade Nitrogen (Airgas, catalog number: NI UHP300 )
- Sodium Dithionite (Sigma-Aldrich, catalog number: 71699 )
- Apiezon High Vacuum grease type L (Fisher Scientific, catalog number: 50-365-186 )
- Nitrogen/argon, gas
- Toluylene blue
- Phenazine methosulfate
- Thionine
- Methylene blue
- Pyocyanin
- Indigotetrasulfonate
- Resorufin
- Indigotrisulfonate
- Indigodisulfonate
- 2-Hydroxy-1,4 naphthoquinone
- Cresyl violet
- Anthraquinone-2,6-disulphonate
- Anthraquinone-2-sulfonate
- Phenosafranine
- Safranine T
- 50 mM potassium phosphate, pH 7.0 (see Recipes)
- Xanthine solution, 10 mM (see Recipes)
- Xanthine oxidase solution (see Recipes)
- Glucose solution, 1 M (see Recipes)
- Catalase solution (see Recipes)
- Glucose oxidase solution (see Recipes)
- Reference dyes solutions (see Recipes)
- Benzyl viologen or methyl viologen (see Recipes)
Equipment
- Spectrophotometer (for example, Agilent 8453 UV-visible spectrophotometer capable of scanning between 300 and 800 nm, with a spectral resolution of 1 nm)
Temperature control is supplied by a Peltier unit from Agilent. Any means to maintain a constant temperature is appropriate (i.e., cuvette holder where the temperature is controlled by a water bath, for example). - An appropriate cuvette to conduct the reaction under strict anaerobic conditions (Figure 2)
Considerations before the reaction:
Choosing an anaerobic cuvette. This method relies on strict anaerobic conditions required for the duration of the reaction. Some labs may be well equipped for this type of study and have a spectrophotometer placed in an anaerobic hood. For many labs, however, this option may not be available, thus the reaction should be well isolated from an aerobic environment by using specialized optical cuvettes (Figure 2). The classical anaerobic cuvette (Figure 2A) has a side arm and air is exchanged by repeated cycles of applying a vacuum and replacing air with nitrogen/argon gas. The xanthine oxidase (XO) which is used to initiate the reaction is placed in the side arm and mixed into the reaction after the cuvette is closed and sealed using vacuum grease. Alternatively, if the specialized cuvette shown in Figure 2A is unavailable, a standard stoppered cuvette (Figure 2B) can be used. Additions are made by a gas tight syringe through an oxygen impermeable septum (Figure 2B, top). The most convenient method which was used by us and others is to use a common stoppered cuvette (Figure 2B, bottom). To achieve anaerobic conditions required for the assay, we use the glucose/glucose oxidase/catalase oxygen scavenging system that efficiently eliminates dissolved oxygen. In addition, the cuvette head space is flushed with a low flow of argon/nitrogen. To initiate the reaction, XO is added with an air tight syringe and after the injection the reaction is carefully mixed with the syringe needle. For some assays when using very low potential flavoproteins or dyes (i.e., a potential less than -300 mV) the argon or nitrogen gas should be additionally treated by an oxygen scrubbing system to remove traces of oxygen. The use of an air tight cuvette (Figure 2A) would be also beneficial. The final volume of the reaction is usually 1 ml but can be scaled up or down depending on the cuvette type and amount of protein to be studied.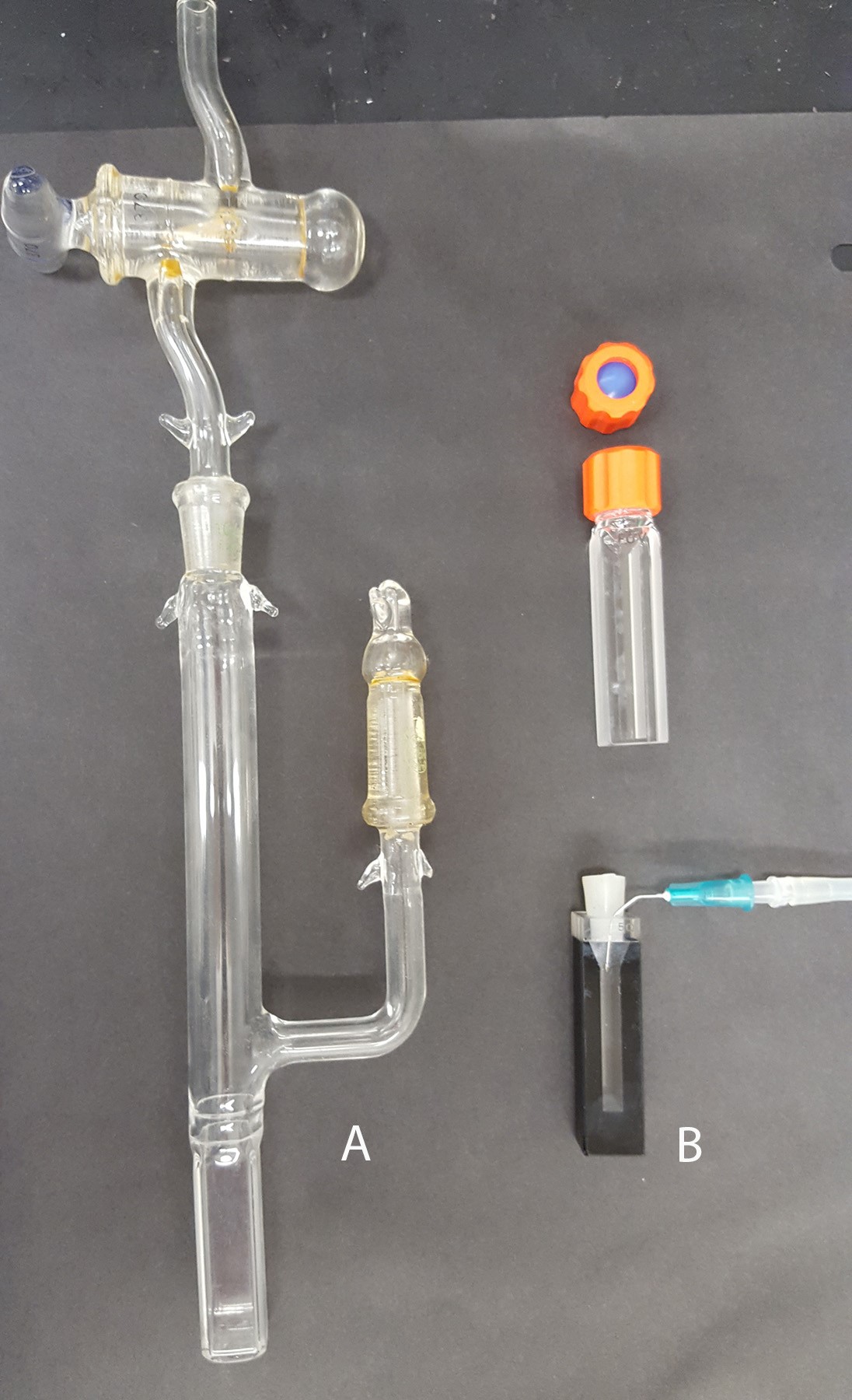
Figure 2. Optical cuvettes that can be used for anaerobic measurements. A. A sealed anaerobic cuvette with a side arm. B. A cuvette with a septum (top) and a standard optical cuvette equipped with a silicone stopper (bottom). Both cuvettes can be used with a gas line to supply argon/nitrogen (shown for the bottom cuvette).
Choosing an appropriate dye. As a general rule, the Em of a reference dye should be within ±30 mV of the anticipated flavin potential in the protein of interest. Table 1 summarized some reference dyes that had been used for determination of flavin reduction potentials. Some tips for choosing a dye: for mutants of a flavoprotein with known potential start with a dye which has an Em closest to the wild-type. For example, a mutation may result in a change of flavin attachment to the protein. Covalently bound flavins demonstrate higher reduction potentials than non-covalent flavins (Heuts et al., 2009). Since wild-type SdhA has a flavin potential of about -50 mV, we used a dye (ITS) which has a redox potential near this value (see Table 1).
Table 1. Reference Dyes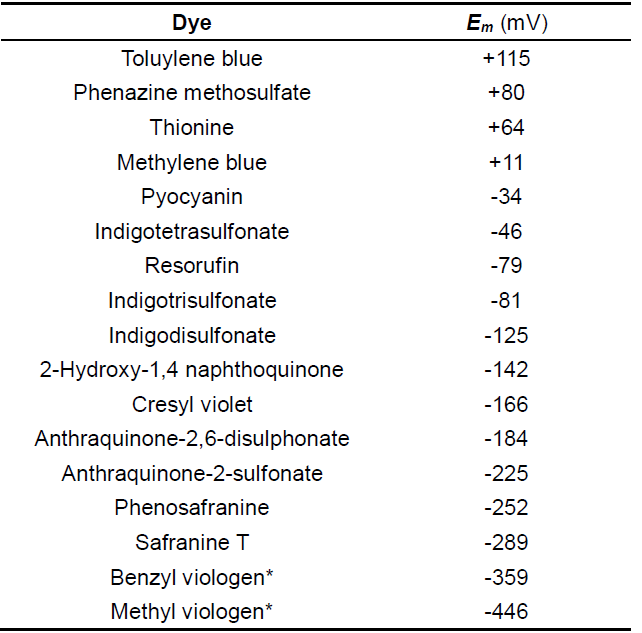
*Viologens are one-electron reduction dyes, all others undergo two-electron reduction.
Choice of the wavelength to monitor absorbance changes in the flavin and dye. As depicted in Figures 1 and 4, the oxidized and reduced forms of the flavin and dye are calculated from the spectra containing both components. Thus, wavelengths used for the analysis should represent where spectral changes are attributed to only one component and where absorbance changes contributed by the second component are negligible. For example, the maximum absorbance of FAD in SdhA is around 460 nm, however, the reduced form of ITS also absorbs at this wavelength and will interfere with correct determination of FAD concentrations. A separate spectrum of dithionite reduced ITS, however, shows that 485 nm is the isosbestic point for ITS (demonstrated in Figure 3). FAD also retains significant absorbance at 485 nm, thus this wavelength can be used for calculations of reduced/oxidized forms of FAD without any contributions from ITS. Reduction of ITS was measured at 614 nm, a peak absorbance, where there is no interference from flavin absorption. Thus, the wavelengths of 485 nm for the flavin and 614 nm for the dye can be used for the analysis.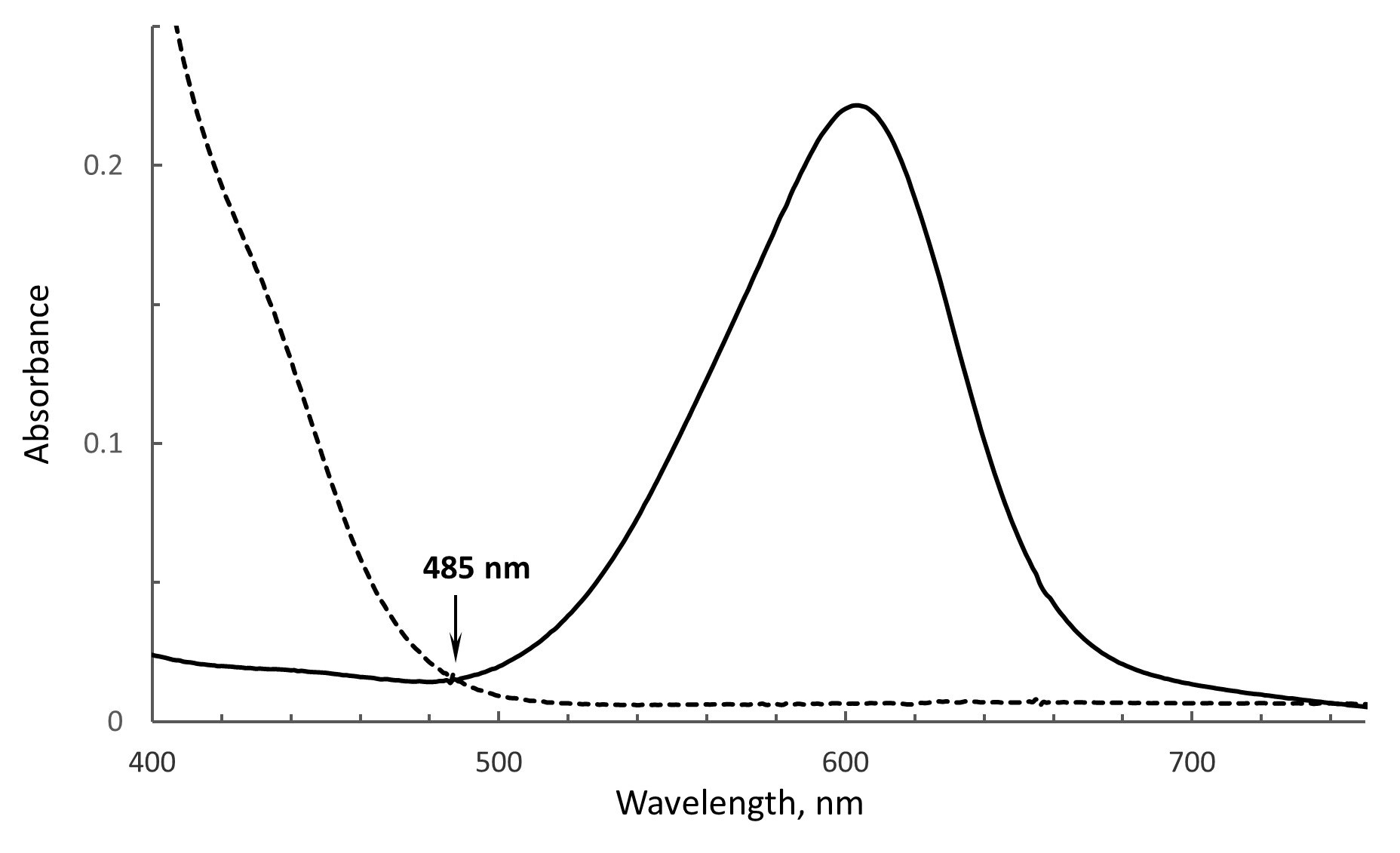
Figure 3. The isobestic point for ITS. The spectrum of oxidized ITS is shown as the solid line and the dithionite reduced ITS is shown as the dashed line. Both forms show the same absorbance at 485 nm, i.e., the isobestic point.
3.An air tight syringe for making additions to the anaerobic assay
Software
- Microsoft Excel or any other spread sheet program for data analysis
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Maklashina, E. and Cecchini, G. (2020). Determination of Flavin Potential in Proteins by Xanthine/Xanthine Oxidase Method. Bio-protocol 10(7): e3571. DOI: 10.21769/BioProtoc.3571.
- Maklashina, E., Rajagukguk, S., Iverson, T. M. and Cecchini, G. (2018). The unassembled flavoprotein subunits of human and bacterial complex II have impaired catalytic activity and generate only minor amounts of ROS. J Biol Chem 293(20): 7754-7765.
分类
生物化学 > 蛋白质 > 定量
分子生物学 > 蛋白质 > 活性
分子生物学 > 蛋白质 > 检测
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link