- EN - English
- CN - 中文
Method for Primary Epithelial Cell Culture from the Rat Choroid Plexus
大鼠脉络丛原代上皮细胞培养方法
(*contributed equally to this work) 发布: 2020年02月20日第10卷第4期 DOI: 10.21769/BioProtoc.3532 浏览次数: 6189
评审: Ehsan KheradpezhouhCristina Isabel CarvalhoAnonymous reviewer(s)
Abstract
The choroid plexus consists of a network of secretory epithelial cells localized throughout the lateral, third and fourth ventricles of the brain. Cerebrospinal fluid (CSF) is generated by the choroid plexus and released into the ventricular environment. This biofluid contains an enriched source of proteins, ions, and other signaling molecules for extracellular support of neurons and glial cells within the central nervous system. Given that other cells in the brain also release factors into the CSF, in vitro investigations of choroid plexus function are necessary to isolate processes selectively occurring within and released from this tissue. Here, we describe a protocol to isolate choroid plexus tissue from each of the ventricular locations, and the cell culture conditions required to support growth and maintenance of these epithelial cells. This technique allows for investigations of the functional significance of the choroid plexus, such as for the examination of stimuli promoting the release of growth factors and extracellular vesicles (e.g., exosomes and microvesicles) from ventricle-specific choroid plexus epithelial cells.
Keywords: Choroid plexus (脉络丛)Background
The choroid plexus produces cerebrospinal fluid (CSF) and maintains chemical homeostasis in the extracellular fluid of the central nervous system. The choroid plexus is comprised of epithelial cells, connective tissue and blood vessels, and this structure is localized in all ventricular regions, including within the lateral, third and fourth ventricles. At each of these sites, the choroid plexus appears to differ relative to structure, function and factors produced and released into the ventricles (Johanson et al., 2005; Lun et al., 2015; Lallai et al., 2019). This tissue actively produces and secretes various signaling molecules, such as growth hormones, transthyretin and transferrin, into the brain and has been implicated in a wide range of functions related to brain development, aging, nutrient transport, endocrine regulation, and pathogenesis of neurodegenerative disorders. As such, dysfunction in the choroid plexus would potentially alter CSF composition and compromise brain health. As the field progresses, the vital function of factors derived from the choroid plexus will certainly continue to emerge, and these advances may then provide a foundation for novel approaches to treat neuropathology in humans.
Previous in vitro approaches to examine choroid plexus function have derived choroidal epithelial cell culture from rat, porcine, human and immortalized murine cells (Zheng and Zhao, 2002; Monnot and Zheng, 2013; Tenenbaum et al., 2013; Delery and MacLean, 2019). However, several considerations have been noted that limit the usefulness of prior techniques. First, immortalized cell lines do not appear to retain the properties of choroid plexus epithelial cells (CPEC) since they become very susceptible to changes in morphology and property in vitro (Angelow et al., 2004). Along these lines, we have found highly variable expression of the choroid plexus specific marker transthyretin across passages in the cell lines Z310 (obtained from Dr. Wei Zheng, Purdue University) and HCPEpiC (commercially available from ScienCell Research Laboratories), suggesting altered protein expression in the immortalized state compared to primary-derived tissues (Lallai and Fowler, unpublished findings). Next, since the location of the epithelial cells used to derive the cell lines may be from one or multiple ventricular locations, and given that different transcript expression and function is found in choroid plexus tissue among the ventricular locations (Lun et al., 2015; Lallai et al., 2019), the immortalized cell lines may not be representative of the specific subregion of the tissue of interest. Indeed, the prior approaches have focused on choroid plexus dissection solely derived from the lateral and/or fourth ventricular locations (e.g., as opposed to the smaller third ventricle), have combined choroid plexus from multiple locations into one sample for analysis, or have focused on species with larger yields of choroid plexus tissue (e.g., primate, porcine and human).
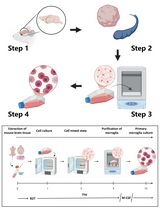
To overcome these challenges, we developed a modified protocol to derive primary culture of choroidal epithelial cells from rodents, which allows one to discern between choroid plexus tissue from third, lateral and fourth ventricles (Lallai et al., 2019). With this approach, we have been able to investigate the release of factors into the cell culture medium, thereby mimicking the physiological release of factors and vesicles into CSF of the brain. Of note, the current protocol also uses exosome-depleted fetal bovine serum (FBS) to allow for examination of extracellular vesicles released from the choroid plexus cells in culture conditions; this condition has been incorporated since non-depleted FBS has been shown to contain a variety of extracellular vesicles containing bovine-derived proteins, RNA and DNA, which may contaminate analyses and conclusions (Wei et al., 2016; Kornilov et al., 2018). Moreover, the cultures obtained from this protocol provide cells with distinct structural characteristics of epithelial cells and express the choroid plexus specific protein transthyretin (Lallai et al., 2019). In the following sections, we describe the methods to discretely dissect choroid plexus tissue from different ventricles and generate primary culture choroidal epithelial cells from rat. Further, recommendations and troubleshooting tips are also provided.
Materials and Reagents
- 0.22 μm filter (Thermo Scientific, catalog number: 595-4520)
- Petri dishes (Fisher Scientific, Diameter 100 mm, catalog number: FB0875712)
- Clear plastic lab wrap (VWR, catalog number: 46610-056)
- Razor blades, straight or double edge (Electron Microscopy Sciences, catalog number: 72003-01)
- 1.7 ml Microtubes, sterile (Olympus Plastics, catalog number: 22-281S)
- P1000 pipet tip (Fisher Scientific, catalog number: 02-707-124)
- Cell culture plates, 96-well (Eppendorf, catalog number: 0030730119)
- Adult Rat to derive brain tissue (Wistar, Charles River, catalog number: Crl:WI 003, or another species/vendor is also acceptable)
- Superglue (Loctite, catalog number: 1647358)
- Laminin (Corning, catalog number: CB-40232)
- Collagenase, Type II (Gibco, catalog number: 17101015)
- Exosome-depleted FBS (SBI System Biosciences, catalog number: EXO-FBS-250A-1)
- 1x TrypLE Express (Gibco, catalog number: 12605-010)
- Poly-D-Lysine (PDL) (Corning, catalog number: CB-40210)
- Isoflurane (Patterson Veterinary, catalog number: 07-893-1389)
- 1x DMEM (Corning, catalog number: 10-017-CV)
- 1x DPBS (Gibco, catalog number: 14040-117)
- Glucose (Fisher Scientific, catalog number: D16-1)
- 1x Pen-Strep (Gibco, catalog number: 15140122)
- 1x HBSS (Corning, catalog number: 21-022-CV)
- Calcium Chloride (Acros Organics, catalog number: 206795000)
- Trypan Blue (Gibco, catalog number: 15250-061)
- Cytosine arabinoside (AraC) (Sigma-Aldrich, catalog number: C1768-1G)
- Cell culture grade sterile water (Corning, catalog number: 25055CV)
- Dissecting Medium (see Recipes)
- 5x Collagenase, Type II Stock Solution (see Recipes)
- 1x Collagenase, Type II Working Solution (see Recipes)
- Choroid Plexus epithelial cells (CPEC) medium (see Recipes)
Equipment
- Rodent guillotine (Harvard Apparatus, catalog number: 73-1918)
- Dissecting brain matrix for rat, coronal plane (Ted Pella, catalog number: 15007)
- Dissecting tools
- Small scissors (Roboz Surgical, catalog number: RS-5840)
- Fine tip forceps (Roboz Surgical, catalog number: RS-4960)
- Tissue forceps (Roboz Surgical, catalog number: RS-8102)
- Microdissection scissors (Roboz Surgical, catalog number: RS-5671)
- Micro Bone Rongeur (Roboz Surgical, catalog number: RS-8306)
- Hemocytometer (Fisher Scientific, catalog number: 0267151B)
- Tally counter (VWR, catalog number: 23609-102)
- Anesthesia machine for isoflurane (E-Z Systems, EZ-150C Classic Vaporizer Machine)
- Isoflurane chamber for rat (E-Z Systems, EZ-178 Mouse/Rat Sure-Seal Induction Chamber)
- Dissecting microscope (Objective for 2-5x magnification, Omano, Stereo Zoom Microscope OM 113-1LP)
- Refrigerated centrifuge (To accommodate 1.7 ml microtubes, Eppendorf, model: 5215R)
- Bead bath for incubation at 37 °C (Fisher Scientific Isotemp Digital-Control Water Bath, model: 205)
- Inverted fluorescent microscope (Objectives for 4x, 10x, and 20x magnification, Life Sciences, Leica DM4000 B LED)
- Cell culture CO2 incubator (Thermo Scientific, Series 8000 Water-Jacketed CO2 Incubators)
- Biosafety hood (NUAIRE, Biological Safety Cabinet Class II Type A/B3)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Lallai, V., Ahmed, A. and Fowler, C. D. (2020). Method for Primary Epithelial Cell Culture from the Rat Choroid Plexus. Bio-protocol 10(4): e3532. DOI: 10.21769/BioProtoc.3532.
- Lallai, V., Grimes, N., Fowler, J. P., Sequeira, P. A., Cartagena, P., Limon, A., Coutts, M., Monuki, E. S., Bunney, W., Demuro, A. and Fowler, C. D. (2019). Nicotine acts on cholinergic signaling mechanisms to directly modulate choroid plexus function. eNeuro 6(2).
分类
神经科学 > 行为神经科学 > 实验动物模型
神经科学 > 细胞机理 > 组织分离与培养
细胞生物学 > 细胞分离和培养 > 细胞分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link