- EN - English
- CN - 中文
Photoactivable Cholesterol as a Tool to Study Interaction of Influenza Virus Hemagglutinin with Cholesterol
利用光活化胆固醇研究流感病毒血凝素与胆固醇相互作用
发布: 2020年02月20日第10卷第4期 DOI: 10.21769/BioProtoc.3523 浏览次数: 4780
评审: David PaulAnonymous reviewer(s)
Abstract
Non-covalent binding of cholesterol to the transmembrane region of proteins affect their functionalities, but methods to prove such an interaction are rare. We describe our protocol to label the hemagglutinin (HA) of Influenza virus with a cholesterol derivative in living cells or with immunoprecipitated protein. We synthesized a “clickable” photocholesterol compound, which closely mimics authentic cholesterol. It contains a reactive diazirine group that can be activated by UV-illumination to form a covalent bond with amino acids in its vicinity. Incorporation of photocholesterol into HA is then visualized by “clicking” it to a fluorophore, which can be detected in an SDS-gel by fluorescence scanning. This method provides a convenient and practical way to demonstrate cholesterol-binding to other proteins and probably to identify the binding site.
Background
Non-covalent interactions of proteins with cholesterol are supposed to regulate trafficking and functionalities of many proteins (de Vries et al., 2015). However, due to the transient and rather weak nature of this interaction, cholesterol-binding proteins and the respective binding sites are notoriously difficult to identify. Highly sophisticated methods, such as NMR or crystallography require large amounts of purified proteins which need to be integrated into artificial lipid membranes and are thus accessible only to specialized labs. A simple procedure involves measuring the cholesterol content in purified proteins using commercial “kits” (e.g., Amplex red cholesterol assay kit, Molecular Probes). However, this method is insensitive and requires solubilization of proteins from membranes with detergent which often removes non-covalently bound lipids.
One improvement is the synthesis of clickable–photocholesterol compounds which can be covalently linked to a protein. They can be added to cells where they are rapidly integrated into membranes and thus can interact with proteins in their native environment. They contain a diazirine group at position 6 of the sterol ring, which disintegrates upon uv-illumination into molecular nitrogen plus a highly reactive carbene-group that forms a covalent bond with amino acid side chains in close vicinity. To detect cross-linked proteins, probes have a latent affinity handle, an alkyne group for chemical conjugation under physiological conditions to azide-reporters by copper-catalyzed azide-alkyne cycloaddition (“click chemistry”). Reporters are either present on “beads” to enrich and identify probe-interacting proteins by mass spectrometry or are fluorophores which allow their in-gel detection.
We synthesized a compound, 6,6'-Azi-25-ethinylcholesterol [termed improved photoclick-cholesterol, complete synthesis is described in Hu et al. (2019)], which is more similar to genuine cholesterol than a commercially available photoclick-cholesterol (Hex-5'-ynyl 3ß-hydroxy-6-diazirinyl-5α-cholan- 24-oate, Avanti Polar lipids, number 700147) since it contains (besides the alkyne group) no further alterations in cholesterol’s alkyl side chain (Figure 1). A previous study showed that 6-photocholesterol is a faithful mimetic of authentic cholesterol (Mintzer et al., 2002). Note, however, that some of the diazirine groups might be photoactivated to other reactive species that have a longer half time than the carbene-group which might unspecifically label proteins. Thus, photocrosslinking is a qualitative rather than a quantitative measure of the cholesterol affinity of a protein. Nevertheless, both cholesterol probes label only a few specific proteins out of all cellular membrane proteins (Thiele et al., 2000; Hulce et al., 2013), indicating that they are suitable tools for analyzing possible interactions between cholesterol and target proteins.
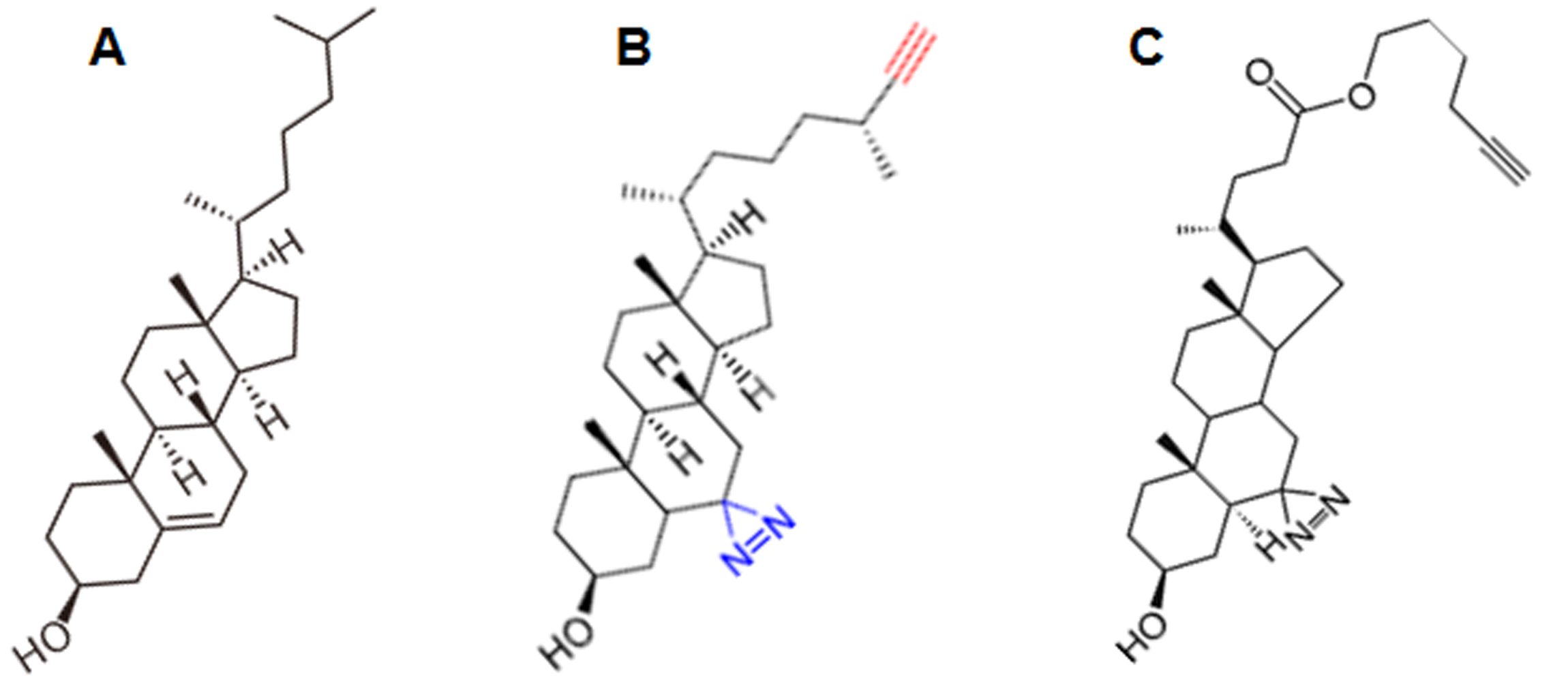
Figure 1. The structure of cholesterol and of two photoactivatable derivatives. A. Cholesterol. B. Improved Photoclick-cholesterol (6,6'-Azi-25-ethinyl-cholesterol) used in this protocol. C. Photoclick-cholesterol (Hex-5'-ynyl 3ß-hydroxy-6-diazirinyl-5α-cholan-24-oate) commercially available from Avanti lipids. The photolabile azide-group is highlighted blue and the alkyne-group in red in B.
Materials and Reagents
- Cell culture consumables
- 6-well/24-well tissue culture plates, flasks, pipettes (Sarstedt, Germany)
- Pipette tips (Sarstedt, Germany)
- Aluminum foil
- CHO-K1 (Chinese hamster ovary cells) (ATCC, catalog number: CCL-61)
- Plasmid (pCAGGS, Niwa et al., 1991)
- DMEM (PAN Biotech, catalog number: P04-04500)
- EDTA-Trypsin (PAN Biotech, catalog number: P10-023100)
- DPBS w/o calcium/magnesium (PAN Biotech, catalog number: P04-36500)
- Penicillin/Streptomycin (10,000 U/ml) (PAN Biotech, catalog number: P06-07100)
- FBS (fetal bovine serum) (PAN Biotech, catalog number: P30-3306)
- Opti-MEMTM I Reduced Serum Medium (Thermo Fisher, GibcoTM, catalog number: 31985070)
- TurboFect Transfection Reagent (Thermo Fisher, Thermo ScientificTM, catalog number: R0531)
- Growth medium (see Recipes)
- Immunoprecipitation
- Ethanol (Sigma, CAS number: 64-17-5, catalog number: 34852-1L-M)
- Tris-HCl (TRIS hydrochloride) (Carl Roth, CAS number: 1185-53-1, catalog number: 9090.3)
- EDTA (AppliChem, CAS number: 6381-92-6, catalog number: A1104)
- Sodium Pyrophosphate (Fisher Scientific, Alfa Aesar, CAS number: 7722-88-5, catalog number: A17546.30)
- Sodium Fluoride (Fisher Scientific, Alfa Aesar, CAS number: 7681-49-4, catalog number: A13019.30)
- Sodium Orthovanadate (Sigma, CAS number: 13721-39-6, catalog number: S6508-10G)
- Benzamidine Hydrochloride (AppliChem, CAS number: 1670-14-0, catalog number: A1380)
- PMSF (AppliChem, CAS number: 329-98-6, catalog number: A0999)
- NEM (N-Ethylmaleimide) (Sigma, CAS number: 128-53-0, catalog number: E3876-5G)
- cOmpleteTM, EDTA-free Protease Inhibitor Cocktail (Sigma, Roche, catalog number: 11873580001)
- NP-40 (Thermo Fisher, Thermo ScientificTM, catalog number: 85124)
- Protein-G-Sepharose 4 Fast Flow (Sigma, GE Healthcare, GE17-0618-01)
- Anti-HA2 antiserum (Hu et al., 2019)
- IP buffer (see Recipes)
- Cell lysis buffer (see Recipes)
- SDS-PAGE and Western blot
- PVDF blotting-membrane, 0.2 µm (VWR, Peqlab, catalog number: 732-3200)
- 1.5 ml microcentrifuge tubes (Sarstedt, Germany)
- 30% acrylamide/ bisacrylamide (37.5:1) (Carl Roth, catalog number: 3029.1)
- Tetramethylethylenediamine (TEMED) (Carl Roth, CAS number: 110-18-9, catalog number: 2367.3)
- Ammonium persulfate (APS) (Carl Roth, CAS number: 7727-54-0, catalog number: 9592.2)
- Sodium dodecyl sulfate (SDS) (Carl Roth, CAS number: 151-21-3, catalog number: 2326.4)
- Bromophenol bule (Sigma, CAS number: 115-39-9, catalog number: 1081220005)
- 1,4-Dithiothreitol (DTT) (Carl Roth, CAS number: 3483-12-3, catalog number: 6908.2)
- Glycerol (Carl Roth, CAS number: 56-81-5, catalog number: 3783.1)
- Skimmed milk powder (Carl Roth, CAS number: 68514-61-4, catalog number: T145.3)
- Tween 20 (Carl Roth, CAS number: 9005-64-5, catalog number: 9127.1)
- PierceTM ECL Plus Western Blotting Substrate (Thermo Fisher, catalog number: 32132)
- Stacking-gel solution (see Recipes)
- Separating-gel solution (see Recipes)
- 5x Non-reducing loading buffer (see Recipes)
- 4x Reducing loading buffer (see Recipes)
- Blocking buffer (see Recipes)
- Crosslinking and click chemistry
- Blacklight Blue (UV) Lamp (Sankyo Denki, power 8W, 3.5 A, 60V, wavelength 320-365 nm, Figure 3A)
- Improved Photoclick-cholesterol (Figure 1B, dissolved in ethanol, the tube is sealed and stored at -20 °C). The long synthesis is described in all details in Hu et al. (2019). But an intermediate alkyne-cholesterol can be purchased from Click Chemistry Tools (https://clickchemistrytools.com/product/alkyne-cholesterol/). With this compound it might be easier to synthesize the final product, the improved Photoclick-cholesterol.
- Picolyl-Azide-Sulfo-Cy3 (Jena Bioscience, catalog number: CLK-1178-1)
- CuAAC Biomolecule Reaction Buffer Kit (THPTA-based) (Jena Bioscience, catalog number: CLK-1178-1)
Equipment
- -20 °C freezer
- Pipette controller (Hirschmann Laborgeräte)
- Heracell 240i CO2 incubator (Heraeus)
- Tabletop centrifuge (Eppendorf, model: 5424R)
- Power pack p25 (Analytik Jena, Biometra P25T)
- Chemiluminescence Imaging, Fusion SL (PeqLab)
- PerfectBlueTM Semi-Dry Electro Blotting Systems (VWR, PeqLab, model: SedecTM M)
- Tube-rotator (Carl Roth, ThermoFisher, model: ATX1.1)
- Typhoon FLA 9400 scanner (GE Healthcare) (Figure 2)

Figure 2. Typhoon FLA 9400 scanner. The gel from Part I Section D, which contains the cross-linked samples and washed with ddH2O, is inserted between two glass plates for scanning with a laser. For results, see Figures 4A and 4B.
Software
- Typhoon Scanner Control v5.0 Software (GE Healthcare)
- ImageQuant (GE Healthcare)
- ImageJ (Fiji)
- Microsoft Excel
- GraphPad Prism
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Hu, B., Gadalla, M. R., Thiele, C. and Veit, M. (2020). Photoactivable Cholesterol as a Tool to Study Interaction of Influenza Virus Hemagglutinin with Cholesterol. Bio-protocol 10(4): e3523. DOI: 10.21769/BioProtoc.3523.
分类
生物化学 > 脂质 > 脂质-病毒互作
生物化学 > 蛋白质 > 相互作用 > 蛋白质-脂质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link