- EN - English
- CN - 中文
Surface Plasmon Resonance Analysis of the Protein-protein Binding Specificity Using Autolab ESPIRIT
利用Autolab ESPIRIT进行表面等离子体共振分析蛋白间结合特异性
发布: 2020年02月20日第10卷第4期 DOI: 10.21769/BioProtoc.3519 浏览次数: 8719
评审: Alka MehraAnonymous reviewer(s)

相关实验方案
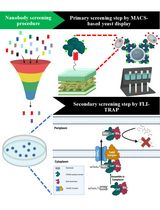
基于蛋白筛选策略从合成文库中分离抗原特异性纳米抗体:结合 MACS 的酵母展示筛选与 FLI-TRAP 方法
Apisitt Thaiprayoon [...] Dujduan Waraho-Zhmayev
2026年01月20日 416 阅读
Abstract
Direct protein-protein interactions are known to regulate a wide range of cellular activities. To understand these contacts one can employ various experimental methods like Dynamic Light Scattering (DLS), Fluorescence Resonance Energy Transfer (FRET), Isothermal titration calorimetry (ITC), Chemical crosslinking, Co-immunoprecipitation (Co-IP), Surface Plasmon Resonance (SPR) and many more. Among these, SPR stands out as a quick, label-free, reliable, and accurate quantitation technique. We have used SPR to elucidate the linkage between 14-3-3 Protein 3 (EhP3) and the actin cytoskeleton in the protist pathogen Entamoeba histolytica. It allowed us to screen EhP3 binding with several actin-binding/actin regulatory proteins (Coactosin, Actophorin, Twinfilin, Profilin, and Filamin). Our screening results suggested Coactosin as an important interacting partner of EhP3. A complete kinetic analysis indeed confirmed that EhCoactosin binds EhP3 with an affinity constant of 3 μM.
Keywords: SPR (表面等离子体共振)Background
Surface Plasmon Resonance (SPR) technique has emerged as one of the most promising screening tools to study macromolecular interactions, since its inception in the early 1990s (Nguyen et al., 2015). It helps determine binding affinities, kinetic parameters, and specificity of these interactions in real-time. This technique is simply based on the optical property of light and principally measures the change in the refractive index upon binding of any molecule to the surface. Surface Plasmon Resonance utilizes the interaction occurring between light and matter. The SPR signal is determined at the surface of the sensor, thus it can be easily correlated to the macromolecules bound on it. This technique captures the real time binding between interacting molecules in a label-free manner, thus making it easy to perform and analyze. Moreover, unlike ITC, this technique is not solution based and uses solid surface for ligand immobilization. SPR uses low quantities of reagents, thus working with small sample size is not an issue.
Principle: Surface Plasmon is a plane-polarized electromagnetic wave that travels on the surface at the interface of the metal coating of the sensor disk (gold disk) and the dielectric medium (sample layer/buffer). These waves are created by continuous fluctuation of charge at the metallic surface. The thin metal surface is placed incident to a laser beam. When the incident light strikes at the interface of the two dielectric media (gold layer and sample layer interface), total internal reflection occurs. Simultaneously, this light generates an evanescent field, with its maximum intensity at the surface of the dielectric material. The resonance occurs when the free electrons of the metal oscillate (plasmon wave) and absorb the plane-polarized light at the specific angle (SPR angle). At the SPR angle, a sharp decrease in the intensity of the reflected light (since some of light is transferred to the Plasmon wave) is observed and this serves as the measurement key. Light passes through a prism and reaches the surface (gold surface) where the molecules are bound. Prism is required to make sure that the wave vector of light in air (kx) is same as the wave vector at the noble metal surface (ksp). The wave vector parallel to the surface is of sole importance in an SPR experiment since the plasmons are confined to the plane of the gold surface. The relationship between kx and incidence angle can be stated as:
kx = k0 nglass sinθinc
where k0 can be calculated as k0 = 2πλ0-1, nglass is the refractive index of the glass prism and θinc is the angle of incidence.
To make the laser light hit the gold disk surface directly, immersion oil is used between the disk and the hemi-cylinder prism (Figure 1). The refractive index of the prism glass, immersion oil, and the disk are same i.e. 1.5. The sensing surface (gold surface) has the lowest refractive index, thus the position of the SPR angle depends on it. Any change (for example: protein-protein interactions typically have a refractive index increment of about 0.18-0.19 ml/g) (Davis et al., 2000) in the dielectric constant or the refractive index changes the resonance angle thus making the SPR-effect a useful tool for us. As the macromolecules bind the surface, the refractive index changes, changing the SPR angle. This change is directly sensed by the detector and translated into response units. The response units obtained are then used to analyze the binding kinetics. The shift in the SPR angle shares a linear relationship with the amount of sample bound. 120 m° (millidegrees) change represents a change in surface protein mass of ~1 ng/mm2. Since light does not penetrate the sample, any colored, turbid, or opaque sample can be used to study.
Figure 1. Pictorial representation of the Surface Plasmon Resonance principle. The diagram depicts the basic underlying principle of the plasmon wave on the gold surface. The Amine groups and the Thiol groups represent the two surface activation method available. The smiley represents the immobilized ligand protein. The chemical structures are labeled to depict the surface chemistry. ksp represents the surface wave vector and kx is the x component of the light wave vector. Absorbed light represents the light at angle of minimum reflection that is recorded as the absorbance by the detector. Evanescent field is a comparable electric field generated by the plasmons on either side of the surface. SPR angle and the Angle of reflection can be clearly seen as read by the detector.
Instrument: Figure 2A shows the Autolab ESPRIT instrument used for the SPR studies. The machine is simple to handle with easy mechanism of operation. The hemi-cylinder is the prism from where the incident light enters and strikes the gold disk. The cuvette (marked as Channel 1 and 2 in the Figure 2B) above it is where all the solutions dispensed in the 384-well plate are injected over the gold disk for the interaction studies to be measured. The syringe pumps modulate the movement of these solutions in and out of the two needles. The Auto sampler moves the needles to and fro for the same. Channel 1 is experimental whereas Channel 2 serves as the control. Simultaneously, the peristaltic pumps below the rack holding the 384-well plate pump in and out the Running buffer. Figure 2C shows the hemi-cylinder with the gold disk placed on it. The Gold Sensor Disk used here is glass disk coated with a very thin layer of gold. The SPR effect can be observed only in the metals where its electrons can behave like a free electron gas. This means that when these electrons move over the surface, their movement is not dependent on the charge that they leave behind. Thus, the only option of metallic surfaces is restricted to Copper, Aluminium, Silver, and Gold.
Figure 2. Autolab ESPRIT instrument. A. The instrument used in the protocol is labeled here. B. Enlarged view of the cuvette, where the reagents are fed onto the gold sensor disk. C. This figure is used from the Autolab manual. The hemi-cylinder is mounted with the gold disks, as placed inside the machine.
Another instrument, commonly used for SPR studies is Biacore T200, sold by G.E. Healthcare. The difference lies at the mechanism of measurement of the resonance angle. Biacore uses a convergent beam, thereby generating a number of incidence and reflecting angles. The disadvantage of this mechanism is that it decreases the resolution to 10-3 in the refractive index. Autolab ESPRIT on the other hand the incident light can be changed by the use of vibrating mirror system. The critical angle for total internal reflection can be changed using the mirrors and the angular shift is measured for a non-coated gold sensor surface with a resolution of approximately 0.02 millidegrees (m°), corresponding to a refractive index resolution of approximately 1 x 10-5. For a coated gold sensor surface, the angular shift is measured with a resolution of approximately 0.1 millidegrees (m°); corresponds to 2 x 10-6 refractive index resolution.
The Chemical steps
Baseline: The first step is to measure the response units for the coupling buffer on the gold sensor disk and is done by simple passing the coupling buffer over the gold surface. It helps in normalization of the further measurements to subtract the noise from the buffer.
Activation: In this step (Figure 3) the gold surface is chemically activated so that protein can be conjugated to it. It can be done by several ways, the most commonly used for example, are: Amine coupling: With the use of EDC (Dimethylaminopropyl-N′-Ethylcarbodiimide N-3-hydrochloride) and NHS (N-Hydroxy Succinimide), the surface can be activated followed by attachment of the protein through its NH2 group. The final concentrations used are 400 mM and 100 mM for EDC and NHS respectively. Thiol coupling: If the ligand protein has a thiol group, then the surface can be made active by using PDEA (2-(2-pyr-idinyldithio) ethaneamine hydrochloride)).
Coupling: At this step, the protein is injected and allowed to bind to the activated surface. This step is termed as immobilization of the ligand protein. As the ligand protein binds, the SPR angle changes linearly with increase in concentration and is measured in its response units.
Blocking: A blocking compound, essentially Ethanolamine here, is added to coat any remaining activated site on the sensor to prevent non-specific binding during the SPR reaction. The blocked sites will be as shown in Figure 1.
Regeneration: This is primarily washing off of any unbound chemical and to set the new baseline value after protein immobilization.
Figure 3. Gold surface activation reactions for the sensor disks. A. The steps for the amine coupling of the ligand protein to the gold surface. B. The steps for the thiol coupling of the ligand protein, using respective chemical reagents. The smiley represents the ligand protein to be immobilized.
Once these steps are successfully performed, the analyte is then passed over the ligand protein to perform the SPR assay. The following Figure 4. represents a typical layout of the reaction and its steps. Here, the running buffer (Association Buffer) is used to set the baseline value since the analyte is prepared in this buffer. As the analyte is injected, the response units increase upon its binding to the ligand protein. Once it reaches a steady state, the dissociation kinetics is measured by just flowing the running buffer over the sensor chip, allowing the analyte to dissociate from the bound ligand. Finally, the regeneration buffer is used to remove the bound analyte to free the ligand protein for the next set of binding reactions.
Figure 4. Steps in a typical SPR assay. Running buffer serves as the baseline for the entire reaction. The Response Units (RU) measured for the running buffer is used to normalize all the calculations. If the injected analyte binds to the ligand protein, then the increase in RU is recorded, while the dissociation of the same analyte leads to the decreasing RU. Regeneration buffer removes any bound analyte (if any) and prepares the ligand for the next round of association reaction.
Relative to other established methods for monitoring protein-protein interactions; SPR-based techniques allow easy experimental designs, rapid testing of hypotheses with high sensitivity, and accurate quantification. In a recent study published by us, SPR was used to determine the interacting partners for EhP3, in order to decipher its connection with the actin cytoskeletal system in Entamoeba histolytica. We conclusively found that EhCoactosin binds EhP3 with an affinity constant of 3 μM (Kumar et al., 2014; Agarwal et al., 2019). This protocol is a one-step handout for anyone who wishes to perform SPR using the Autolab ESPRIT machine. We have explained all the steps in an elaborate manner to help the user to carryout a hassle free experiment. The mechanisms of this instrument are different from Biacore T200 and this protocol can be easily followed by, even a first time user.
Materials and Reagents
- Bare gold sensor disk (Metrohm Autolab, catalog number: 1-04-04-000)
- 384-well Clear Flat Bottom Polystyrene Not Treated Microplate, with Lid, Sterile (Corning, catalog number: 3680)
- 6-well Clear Not Treated Multiple Well Plates (Corning Costar, catalog number: 3736)
- Immersion oil
- Demineralized (demi) water (Sigma-Aldrich, catalog number: 38796)
- Mercaptoundecanoic acid (11-MUA) (Sigma-Aldrich, catalog number: 450561, MW 218.36 g/Mol)
- N-Hydroxy Succinimide (NHS) (Sigma-Aldrich, catalog number: 130672, MW 115.09 g/Mol)
- Dimethylaminopropyl-N′Ethylcarbodiimide N-3-hydrochloride (EDC) (Sigma-Aldrich, catalog number: 03450, MW 191.70 g/Mol)
- Ethanolamine (Sigma-Aldrich, catalog number: E6133, MW 97.54 g/Mol)
- Sodium acetate trihydrate (Sigma-Aldrich, catalog number: S8625, MW 136.08 g/Mol)
- Acetic acid (Sigma-Aldrich, catalog number: A6283)
- HEPES sodium salt (Sigma-Aldrich, catalog number: H7006, MW 260.29 g/Mol)
- Ethanol, ≥ 99.9% (Merck, catalog number: 100983)
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653, MW 58.44 g/Mol)
- Sodium hydroxide (NaOH) (Merck, catalog number: 1.06498, MW 40.00 g/Mol)
- Hydrochloric acid (HCl), 37% (Merck, catalog number: 100317)
- Tween 20 (Sigma-Aldrich, catalog number: P1379)
- Nitrogen gas (Sigma-Aldrich, catalog number: 295574)
- 11-MUA solution (see Recipes)
- 400 mM EDC (see Recipes)
- 100 mM NHS (see Recipes)
- 1 M Ethanolamine (see Recipes)
- 1 M Acetate buffer (see Recipes)
- 1 M HEPES buffer (see Recipes)
- 5 M NaCl (see Recipes)
- 1 M NaOH (see Recipes)
- 10% Tween 20 (see Recipes)
- Buffer 1: Coupling buffer (see Recipes)
- Buffer 2: Association buffer (see Recipes)
- Buffer 3: Regeneration buffer (see Recipes)
- Protein solutions (see Recipes)
Equipment
- Tweezers
- Pipettes
- Autolab SPR ESPRIT (Metrohm, model: Autolab ESPRIT)
- Tabletop high-speed centrifuge (Eppendorf, model: 5430R)
- Filtration unit (Milipore, model: XX1514700, and simple Syringe filters)
Software
- Autolab Data Acquisition program
- Autolab Kinetic Evaluation program
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Rath, P. P., Anand, G. and Agarwal, S. (2020). Surface Plasmon Resonance Analysis of the Protein-protein Binding Specificity Using Autolab ESPIRIT. Bio-protocol 10(4): e3519. DOI: 10.21769/BioProtoc.3519.
分类
分子生物学 > 蛋白质 > 蛋白质-蛋白质相互作用
生物物理学 >
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link