- EN - English
- CN - 中文
Transcervical Mouse Infections with Chlamydia trachomatis and Determination of Bacterial Burden
小鼠宫颈沙眼衣原体感染及细菌负荷测定
发布: 2020年02月05日第10卷第3期 DOI: 10.21769/BioProtoc.3506 浏览次数: 5908
评审: Alka MehraFrank FollmannAchille Broggi
Abstract
Chlamydia trachomatis is an obligate human pathogen. It infects the genital tract of humans ascending into the fallopian tube, exacerbated by chronic pelvic pain, pelvic inflammatory disease, and fallopian tube scaring resulting in infertility and other malignancies. The major hurdle in controlling chlamydial spread is that the infection remains asymptomatic, thus leading to chronic, recurrent and persistent infections, with no vaccines developed so far. Being a human pathogen, we do not have an in vivo model of C. trachomatis infection. C. trachomatis do not cause ascending infections and fallopian tube pathology in the mouse urogenital tract when infected vaginally. To overcome this hurdle trans cervical method of infection must be adapted. In this protocol the method of establishing trans-cervical chlamydial infection with the procedure to determine the bacterial load is detailed. This method will facilitate to deliver the bacteria past the cervix establishing an ascending infection into the uterine horns reciprocating human fallopian tube infections.
Keywords: Chlamydia trachomatis (沙眼衣原体)Background
Sexually transmitted infections (STI) remain major societal and economic burden despite public health initiatives, vaccinations and development of antibiotics (O’Connell and Ferone, 2016). There is estimated to be 357 million new infections annually and STIs have been identified by the WHO as a global priority for elimination (WHO Global Health Sector Strategy on Sexually Transmitted Infections 2016-2021). Among the various bacterial STI, Chlamydia trachomatis is the most common STI (Starnbach, 2018) and stands first with a 131 million new infections annually worldwide (Poston et al., 2017). High reinfection in the genital tract is exacerbated by chronic pelvic pain, pelvic inflammatory disease, and fallopian tube scaring (O’Connell and Ferone, 2016) resulting in infertility and other malignancies. The major hurdle in controlling chlamydial spread is that the infection remains asymptomatic, thus leading to chronic, recurrent and persistent infections, with no vaccines developed so far.
Chlamydia undergoes a unique, biphasic developmental cycle that generally modulates between two morphological forms. Extracellular, infectious elementary bodies (EBs) attach to host cells within 15 min of infection, after which they are internalized into a membrane bound vesicle called an inclusion. EBs then differentiate into metabolically active, non-infectious, reticulate bodies (RBs) in 12 h that undergo binary fission, followed by secondary differentiation into EBs after 36 h post infection (hpi). Within/after 48 hpi most of the bacteria is in the EB form and are released upon host cell lysis or extrusion (Abdelrahman and Belland, 2005).
Chlamydia trachomatis being an obligate human pathogen lacks an in vivo model. To evaluate specific vaccine antigens that can be used in humans, and to study the host pathogen interaction to understand the pathogenicity of Chlamydia infection, a suitable in vivo model need to be established (De Clercq et al., 2013). Unlike in Chlamydia muridarium, a natural pathogen in mouse, intravaginal inoculation of the bacteria only leads to a mild genital infection and does not cause ascending infection or tubal pathology. The innate immune response in the lower genital tract of the mouse can rapidly eliminate human chlamydial organism (Sturdevant and Caldwell, 2014). Thus a higher number of infectious particles are required to establish infection, which yields a 2 log units lower bacterial load in the uterine horns (Darville et al., 1997; Ramsey et al., 2000). To address this limitation an intra-bursal mode of infection was established (Carmichael et al., 2013), via a survival surgery and directly inoculating the bacteria into the bursal region. This method bypasses both the cervical barrier and endometrial lumen, thus do not resemble the natural path of vaginal infection (see Figure 1).
The primary site of Chlamydia trachomatis infection is the cervical epithelium (Buckner et al., 2016). Subsequently, the infection ascends to the upper genital tract tissues, the uterine horns and oviducts, which frequently lead to hydrosalpinx, fibrosis, and infertility, which are also common post-infection sequelae in women (Morrison and Caldwell, 2002; Shah et al., 2005). Mice have a bicornuate uterus with two lateral horns and are lined with ligaments carrying blood and lymphatic vessels. The cervix has cranial, fundal and caudal segments. The caudal segment or cervix consists of a single cavity that protrudes into the vaginal opening. To establish an ascending infection, which closely resembles infection in human uterus and fallopian tube, we need to bypass the cervix and inoculate the pathogen into the uterine fundus. This leads to an easy establishment of ascending infection in mouse. This method has been made used in various study (Gondek et al., 2012; Pal et al., 2018; Rajeeve et al., 2018).
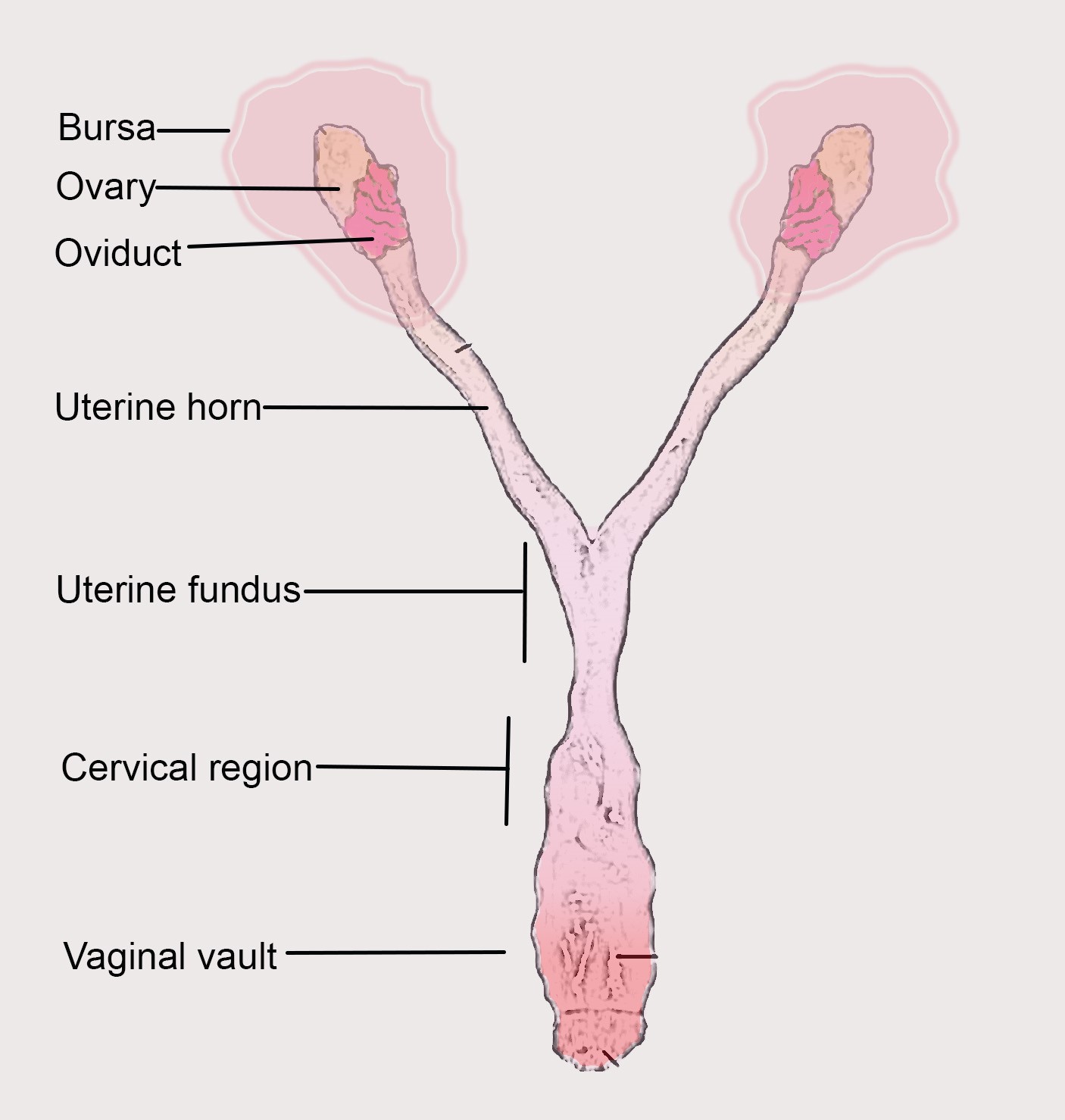
Figure 1. Mouse reproductive tract with parts labelled
Materials and Reagents
Transcervical infection and DNA extraction
- Eppendorf tubes (Stastedt GmbH, catalog number: 72.688.004)
- Ultracentrifugation tubes (Beckman Coulter, catalog number: NC9146666)
- 15 ml Falcons (Corning, catalog number: 430791)
- 50 ml Falcon
- 12-well and 24-well plate
- Rubber scraper
- 75 cm2 or 150 cm2 flasks (Corning, catalog numbers: CLS3290 and CLS3291)
- Nitrile glove (Microflex Neotouch, catalog number: 93-833)
- Sterile 200 μl pipette tips (Stastedt GmbH, catalog number: 25-201)
- 1 ml syringe (BD Leur-LokTM, catalog number: 309628)
- NSET: Non-surgical embryo transfer device (ParaTechs Corp, Product number: 60010)
- Post-pubertal and sexually mature (> 42 days old) female mice (any strain)
- Medroxy-progesterone acetate (Depo-provera R 250 mg tablets; Hexal)
- Chlamydia trachomatis L2 stock (1 x 107 bacteria: ATCC VR-902 B)
- RPMI Media 1640+ GlutaMax (Gibco, catalog number: 11875)
- Heat inactivated Fetal Calf Serum (FCS) (Sigma, catalog number: F2442)
- DNeasy blood and tissue kit (Qiagen, catalog number: 69504)
- Tissue homogenization beads (MP Biomedicals, catalog number: 6913-500)
- 1x DPBS (Gibco, catalog number: 14190)
- Sucrose (Sigma, catalog number: S0389)
- Glutamic acid (Sigma, catalog number: G1251)
- Nuclease free water (Sigma, catalog number: 7732-18-5)
- SYBR green (Thermo Scientific, catalog number: A25741)
- Primers (Sigma)
- Meglumin diatrizoate (Sigma, CAS: 131-49-7)
- Sodium diatrizoate hydrate (Sigma, CAS: 737-31-5)
- Sodium Citrate dehydrate (Sigma, catalog number: 106447)
- EDTA (Sigma, CAS: 60-00-4)
- HBSS (Sigma, catalog number: H4385)
- Renografin
- 10 mM sodium phosphate (8 mM Na2HPO4 and 2 mM NaH2PO4; Sigma, CAS: 7558-79-4; CAS: 7558-80-7)
- SPG buffer (see Recipes)
- Renografin (see Recipes)
Equipment
- Pipettes
- Incubator (ThermoScientific)
- Centrifuge (Beckman Coulter, model: AvantiTMJ 25I)
- Ultra centrifuge (Beckman Coulter, model: Optima L-80 XP)
- -80 °C freezer (New Brunswick Scientific)
- RT-PCR instrument (ABI 7500)
Software
- Microsoft Excel
- Step One Plus software package (ABI 7500)
- GraphPad Prism 7
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Rajeeve, K. and Sivadasan, R. (2020). Transcervical Mouse Infections with Chlamydia trachomatis and Determination of Bacterial Burden. Bio-protocol 10(3): e3506. DOI: 10.21769/BioProtoc.3506.
分类
微生物学 > 微生物-宿主相互作用 > 细菌
免疫学 > 动物模型 > 小鼠
分子生物学 > DNA > DNA 定量
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link