- EN - English
- CN - 中文
Protocols for Processing and Interpreting cryoEM Data Using Bsoft: A Case Study of the Retinal Adhesion Protein, Retinoschisin
利用Bsoft处理和解释冷冻电镜数据方法:视网膜粘连蛋白,视网膜劈裂蛋白的实例研究
发布: 2020年01月20日第10卷第2期 DOI: 10.21769/BioProtoc.3491 浏览次数: 4730
评审: Edgar Soria-GomezBeatrice LiAnonymous reviewer(s)
Abstract
The goal of cryoEM is to determine the structures of biomolecules from electron micrographs. In many cases the processing is straightforward and can be handled with routine protocols. In other cases, the properties and behavior of the specimen require adaptions to properly interpret the data. Here I describe the protocols for examining the higher order assemblies of the retinal adhesion protein, retinoschisin (RS1), using the Bsoft package. The protocols for micrograph preprocessing, 2D classification and 3D alignment and reconstruction follow the usual patterns for the majority of cryoEM specimens. The interpretation of the results is specific to the branched network of RS1 filaments. The 2D class averages are used to determine the relative positions of the RS1 molecules, thus defining the interacting interfaces in the network. The major interface of the linear filament is then further examined by reconstructing the “unit cell” and fitting the molecular models.
Keywords: Electron microscopy (电子显微镜)Background
In cryoEM, the aim is to determine the structures of biomolecules to obtain biologically relevant information. The usual operation is to align, classify and average the 2D particle images obtained from electron micrographs, as well as align and reconstruct these images in 3D. There are several different approaches and many software packages that accomplish these tasks (https://en.wikibooks.org/wiki/Software_Tools_For_Molecular_Microscopy). The one I developed and used here is Bsoft (Heymann, 2001; Heymann and Belnap 2007; Heymann et al., 2008; Heymann, 2018a and 2018c).
In addition to the normal processing protocols, I describe how to approach a specific example, retinoschisin (RS1) (Tolun et al., 2016; Heymann et al., 2019), that exhibits behavior that requires specialized treatment for interpretation (Figure 1A). RS1 forms a network of filaments that adhere to the air-water interface (Figures 1B-1D). Biomolecules typically locate to the air-water interface (Noble et al., 2018; Noble et al., 2019), and often present a preferred orientation that complicates 3D reconstruction. The RS1 molecules in the filaments mainly present only one view. However, these still show many interactions between the individual molecules. Therefore, the strategy is to extract images large enough to contain several RS1 molecules, perform 2D classification and examine the prevalent interaction types (Figure 1A) (Heymann et al., 2019). To deal with the preferred orientation, a partial solution is to process micrographs of 30° tilted specimens to obtain a 3D map of the most common interaction. The 3D reference used here is a “unit cell” containing two RS1 molecules of the linear filament. Two molecules of RS1 are then fitted into the map to examine the interacting residues at their interface.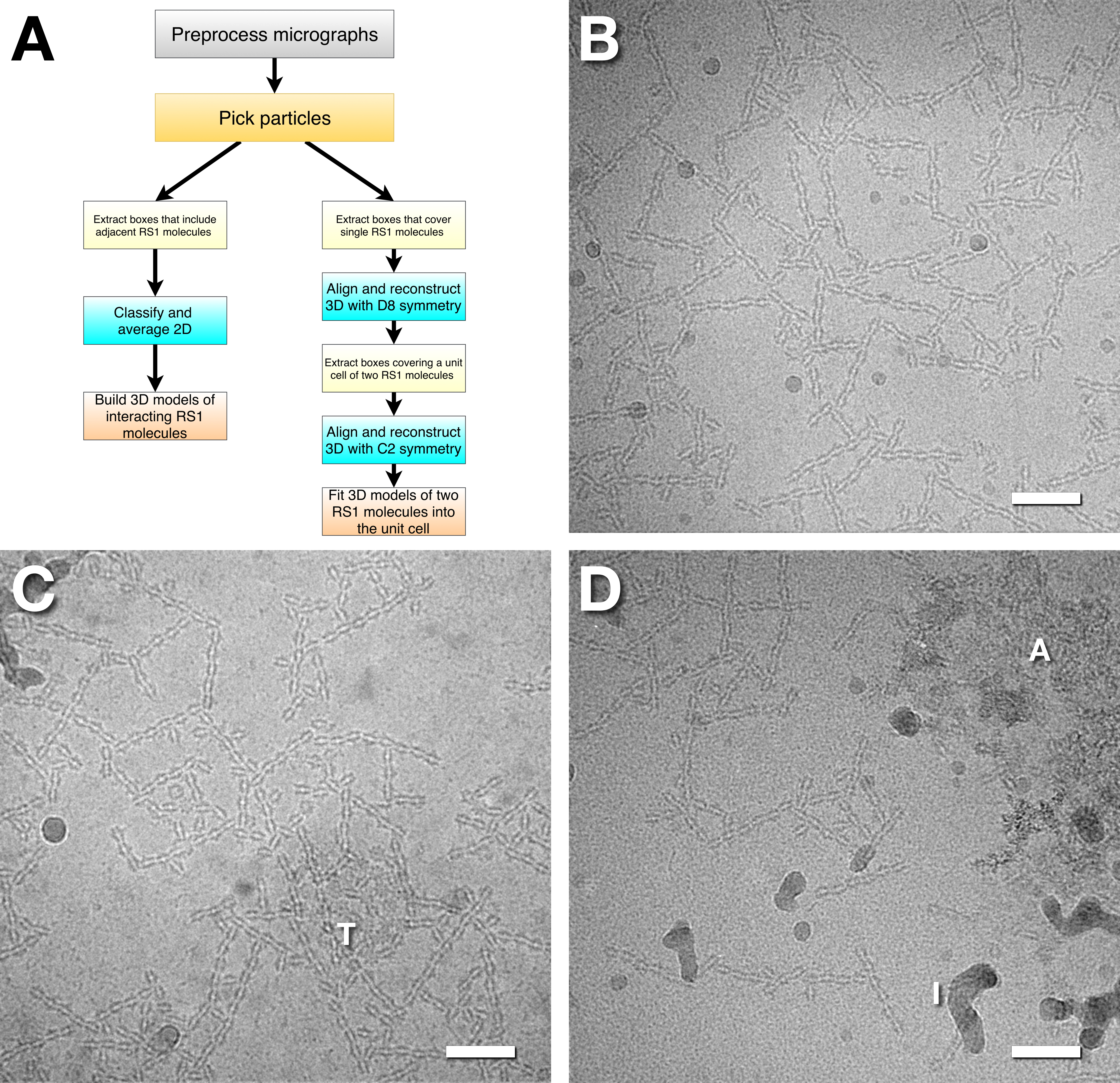
Figure 1. Protocol scheme and examples of micrographs of RS1. A. The protocol scheme starts with two processing sections common to cryoEM, followed by either 2D classification and averaging or 3D alignment and reconstruction. The results are then interpreted by modeling the interactions between the RS1 molecules. B. A good micrograph, showing a gradient of intensity from the top left to the bottom right. The intensity indicates the specimen thickness, where the lighter parts are thinner and more suitable for particle picking. C. A reasonable micrograph with some ice crystals. The “T” shows a cluster of top views of RS1 located in a somewhat thicker region. D. A micrograph with crystalline ice (“I”) and aggregated RS1 (“A”). Scale bars = 500 Å.
Equipment
- Computational setup
The computational work I describe can be done on any UNIX-based platform, such as Linux and MacOSX. All programs in the Bsoft package (Heymann, 2018a) can run on a single machine with multiple CPU cores. However, single particle analysis is computationally intensive and typically requires a cluster to run in a reasonable amount of time. The computational power needed scales with the number and size of particle images, as well as the choice of reference and processing parameters. In this particular case, I used approximately 900 CPU cores (Linux and MacOSX) in our cluster for the following tasks:- Micrograph preprocessing: ~70 h for 1,354 micrographs, each with 50-54 frames.
- 2D alignment and classification of 39,536 particles of size 330 x 330: ~12 h over five iterations.
- 3D alignment of 26,702 particles of size 168 x 168: ~10 h over six iterations.
- 3D alignment of 26,702 particles of size 224 x 224: ~4 h over four iterations.
Software
- Bsoft basics
All of the processing I describe here can be done with the current version of Bsoft (version 2.0.6) (Heymann, 2018a).- brun
Bsoft contains a large number of command-line programs. The program brun can be used to launch these command line programs in a more interactive way. Typing any program name on the command line (without options) provides usage information, including all the options. The options for these programs can be abbreviated, as long as they are unique. All the programs have a “-verbose” option that sets the level of information generated. Without this option, no information will be generated. With quick tasks the verbosity level is typically set to 7, which will give image statistics and parameters for the operation being executed. With extensive tasks (such as aligning particle images or performing reconstructions), the verbosity is usually set to 1 to limit the information to the most important results. - bshow
The main program for interactive work is bshow. It presents the user with an image processing environment where many different operations can be performed. For some programs there are options to plot the output data in Postscript files. These are text files with the data encoded as tables that can be easily imported into other plotting programs for better visualization. The conventions in Bsoft adhere to those we specified in 2005 (Heymann et al., 2005). Because one can use operations other than Fourier transformation to calculate frequency terms, I use the term “frequency space” rather than “Fourier space”. Many of the programs in Bsoft have been parallelized using Grand Central Dispatch on Mac OSX and OpenMP on Linux and will automatically use as many cores as available. In some cases the user can specify the number of threads (i.e., cores) to use (e.g., breconstruct). The Bsoft package (Heymann, 2018a) is freely available at http://bsoft.ws.
- brun
- Distributed processing
The command line programs in Bsoft are executable in any Unix-like environment and from any commonly used script language (such as bash, perl and python). Because each particle is aligned on its own, the philosophy is to separate a set of particles into smaller subsets and run those on different machines. This means that one can use a heterogenous mixture of computers to distribute the tasks. We use the Peach distributed processing system (Leong et al., 2005), but the same can be done on any cluster with a proper queueing manager. - Validation as an integral part of the processing
The success and validity of all micrograph processing depends on understanding the influence of noise and how to avoid bias imposed by user decisions. Most of the principles are laid out in Heymann (Heymann, 2018a). The best practices involve three recommendations:- Process at least two independent sets of particle images–I typically split the set of micrographs into manageable subsets before processing.
- Use conservative resolution limits during particle alignment.
- Test the final reconstruction for coherency: i.e., whether the averages or reconstructions from particle images are better than from noise images.
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Heymann, J. B. (2020). Protocols for Processing and Interpreting cryoEM Data Using Bsoft: A Case Study of the Retinal Adhesion Protein, Retinoschisin. Bio-protocol 10(2): e3491. DOI: 10.21769/BioProtoc.3491.
- Heymann, J. B., Vijayasarathy, C., Huang, R. K., Dearborn, A. D., Sieving, P. A. and Steven, A. C. (2019). Cryo-EM of retinoschisin branched networks suggests an intercellular adhesive scaffold in the retina. J Cell Biol 218(3): 1027-1038.
分类
生物化学 > 蛋白质 > 成像
生物物理学 > 显微技术 > 低温显微镜技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link