- EN - English
- CN - 中文
Studying Protein Aggregation in the Context of Liquid-liquid Phase Separation Using Fluorescence and Atomic Force Microscopy, Fluorescence and Turbidity Assays, and FRAP
利用荧光和原子力显微镜、荧光和浊度分析以及FRAP研究液-液相分离中的蛋白质聚集
发布: 2020年01月20日第10卷第2期 DOI: 10.21769/BioProtoc.3489 浏览次数: 9511
评审: Ralph Thomas BoettcherBrahma MuluguAnonymous reviewer(s)
Abstract
Liquid-liquid phase separation (LLPS) underlies the physiological assembly of many membrane-less organelles throughout the cell. However, dysregulation of LLPS may mediate the formation of pathological aggregates associated with neurodegenerative diseases. Here, we present complementary experimental approaches to study protein aggregation within and outside the context of LLPS in order to ascertain the impact of LLPS on aggregation kinetics. Techniques described include imaging-based approaches [fluorescence microscopy, atomic force microscopy (AFM), fluorescence recovery after photobleaching (FRAP)] as well as plate reader assays [Thioflavin-T (ThT) fluorescence intensity and turbidity]. Data and conclusions utilizing these approaches were recently reported for the low complexity domain (LCD) of the transactive response DNA binding protein of 43 kDa (TDP-43).
Keywords: Liquid-liquid phase separation (液相分离)Background
Many proteins that aggregate in neurodegenerative disease also undergo LLPS to form dynamic, reversible liquid-like droplets (Molliex et al., 2015; Conicella et al., 2016; Hofweber et al., 2018). LLPS is commonly mediated by the presence of intrinsically disordered regions (IDRs) that enable transient, weak, multivalent interactions (Lin et al., 2017). These IDRs are often composed of only a few amino acid types and may be arranged in short, repetitive motifs. The low complexity domain (LCD) of TDP-43 forms pathologically-associated amyloid aggregates and was recently shown to phase separate (Conicella et al., 2016; Lim et al., 2016). However, any relationship between these two processes remained unclear. We recently demonstrated that amyloid formation by the LCD can occur within the context of LLPS (Babinchak et al., 2019). In this work, we implemented a combinatorial experimental approach to assess the kinetic role of LLPS in the formation of amyloids that can be applied to other aggregation-prone proteins that phase separate (Figure 1). This approach includes four major components: (1) imaging of amyloid and droplet-like species using fluorescence microscopy and atomic force microscopy (AFM); (2) assessment of LLPS propensity using turbidity measurements; (3) monitoring amyloid aggregation via Thioflavin-T (ThT) fluorescence intensity measurements; and (4) assessment of maturation of liquid-like droplets using fluorescence recovery after photobleaching (FRAP). Analysis of these results can provide quantitative insights for comparing aggregation under LLPS conditions and in the absence of LLPS.
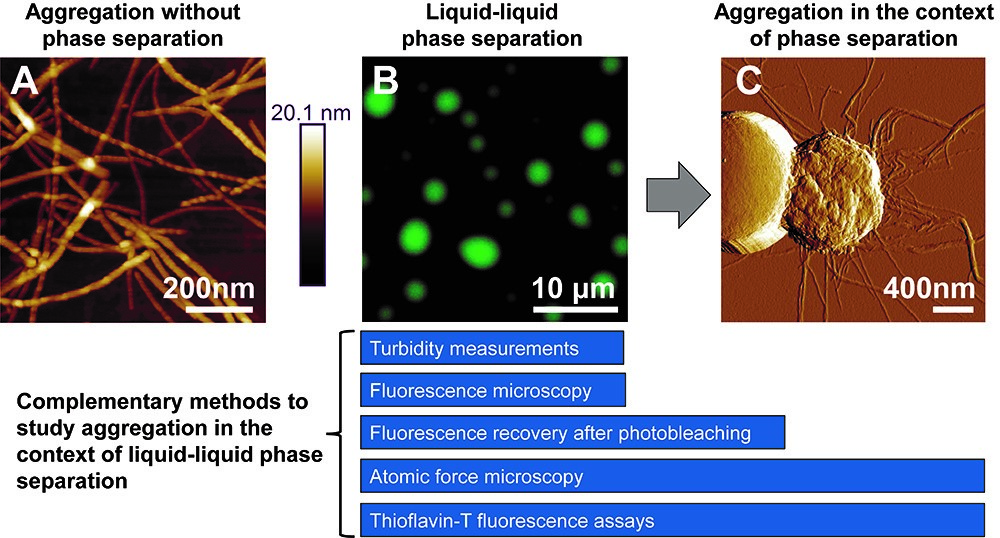
Figure 1. Methods for studying the formation of TDP-43 LCD amyloid aggregates from liquid-like droplets. A. Atomic force microscopy (AFM) imaging of amyloid aggregates formed by the TDP-43 LCD in the absence of LLPS, which often appear as individual, non-clumped fibers. B. Fluorescence microscopy of liquid-like droplets formed by the TDP-43 LCD. C. With time, fibrils emanating from within mature droplets can be observed on AFM imaging and fibrils often appear clumped or intertwined. Complementary methods for studying aggregation within the context of liquid-liquid phase separation are presented. Turbidity measurements and fluorescence microscopy are optimal methods for studying liquid-like droplets, while ThT assays can be used to study amyloid formation. The transition from liquid-like droplets to amyloids can be captured using a combination of FRAP, AFM, and ThT assays. All images were originally published in J. Biol. Chem. (Babinchak et al., 2019).
Materials and Reagents
- Pipette tips
- FluoroDish Cell Culture Dish (35 mm dish, 23 mm well, glass thickness: 0.17 mm) (World Precision Instruments, catalog number: FD35-100)
- Amicon® Ultra–0.5 ml Centrifugal Filters (Ultracel–100,000 nominal molecular weight limit, NMWL) (Millipore, catalog number: UFC510096)
- ScotchTM Tape
- Adhesive tabs (Ted Pella, Inc., catalog number: 16079)
- AFM specimen discs, 15 mm diameter (Ted Pella, Inc., catalog number: 16218)
- Mica discs, 9.9 mm diameter (Ted Pella, Inc., catalog number: 50)
- Corning® Assay Plate, 96-well half area (non-treated, no lid; black with clear flat bottom; polystyrene) (Corning, catalog number: 3880)
- Sealing Tape, Advanced Polyolefin (Certified DNase-, RNase-, and Nucleic Acid-free) (Thermo ScientificTM, catalog number: 235307)
- Millex®–GV filter unit, low protein binding Durapore® (PVDF) membrane, 0.22 μm (Millipore, catalog number: SLGV033RS)
- Zeba spin desalting column (2 ml; 7 kDa molecular weight cut off) (Thermo ScientificTM, catalog number: 89890)
- Microscope Cover Glass 12CIR-1 (Thermo ScientificTM, catalog number: 1254580)
- Alexa Fluor 488TM C5 Maleimide (InvitrogenTM, catalog number: A10254)
- Potassium phosphate monobasic, KH2PO4 (Fisher Scientific, catalog number: 7778-77-0)
- Potassium phosphate dibasic, K2HPO4 (Fisher Scientific, catalog number: 7758-11-4)
- Sodium acetate trihydrate, CH3COONa·3H2O (Fisher Scientific, catalog number: 127-09-3)
- Acetic acid (glacial), CH3COOH (Fisher Scientific, catalog number: A38-212)
- Urea (Fisher Scientific, catalog number: BP 169-500)
- NaCl, 5 M solution (Sigma, catalog number: S5150-1L)
- Imidazole (Fisher Scientific, catalog number: O3196)
- Thioflavin-T (Sigma-Aldrich, catalog number: T-3516)
- Tris (2-carboxyethyl) phosphine hydrochloride, TCEP (GoldBio, catalog number: TCEP25)
- Trizma Base, Tris (Sigma-Aldrich, catalog number: T1503)
- Hydrochloric acid, HCl (Fisher Scientific, catalog number: A144)
- Type F immersion liquid (Leica Microsystems, catalog number: 11513859)
- Silicon tip on nitride lever probe (Bruker, SCANASYST-AIR)
- Milli-Q H2O (from Milli-Q Reference Water Purification System, MilliPore Sigma)
- 100 mM Phosphate and Acetate Stock Buffers (10x) (see Recipes)
- Thioflavin-T stock buffer (see Recipes)
- Labeling buffer (see Recipes)
- Equilibration buffer (see Recipes)
Equipment
- Plate Reader (Tecan Life Sciences, model: Infinite M1000)
- Pipette
- EppendorfTM Microcentrifuge (Fisher Scientific, model: 5424)
- Computer
- Vortex-Genie 2 Lab Mixer (Scientific Industries, Inc., model: G-560)
- pH/mV meter (Fisher Scientific, model: Accumet AB150)
- NanoDropTM Spectrophotometer (Thermo ScientificTM, model: 2000)
- Leica TCS SP8 confocal microscope (Leica Microsystems)
- All-in-one fluorescence microscope (Keyence, model: BZ-X710)
- 100x PlanApoλ objective (NA 1.45, oil immersion) (Nikon, catalog number: MRD31905)
- GFP filter cube (Keyence, catalog number: OP-87763)
- Multimode Atomic Force Microscope with Nanoscope V (Bruker)
- Incubator (Fisher Scientific, model: 516D)
Software
- i-controlTM 1.10 (for infinite reader) software (Tecan Life Sciences)
- Leica Application Suite X (LAS X, Leica Microsystems)
- Nanoscope 9.1 (Bruker)
- Nanoscope Analysis 1.5 (Bruker)
- BZ-X Viewer (Keyence)
- BZ-X Analyzer (Keyence)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Babinchak, W. M. and Surewicz, W. K. (2020). Studying Protein Aggregation in the Context of Liquid-liquid Phase Separation Using Fluorescence and Atomic Force Microscopy, Fluorescence and Turbidity Assays, and FRAP. Bio-protocol 10(2): e3489. DOI: 10.21769/BioProtoc.3489.
- Babinchak, W. M., Haider, R., Dumm, B. K., Sarkar, P., Surewicz, K., Choi, J. K. and Surewicz, W. K. (2019). The role of liquid-liquid phase separation in aggregation of the TDP-43 low-complexity domain. J Biol Chem 294(16): 6306-6317.
分类
生物物理学 > 显微技术 > 原子力显微镜
神经科学 > 神经系统疾病 > 神经退行性病变
生物化学 > 蛋白质 > 自组装
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link