- EN - English
- CN - 中文
Quantifying HIV-1-Mediated Gut CD4+ T Cell Death in the Lamina Propria Aggregate Culture (LPAC) Model
固有层聚集培养模式中HIV-1介导的Gut CD4+ T细胞凋亡的量化分析
(*contributed equally to this work) 发布: 2020年01月20日第10卷第2期 DOI: 10.21769/BioProtoc.3486 浏览次数: 5038
评审: Gal HaimovichRajesh ThippeshappaAnonymous reviewer(s)

相关实验方案
来自骨髓增生性肿瘤患者的造血祖细胞的血小板生成素不依赖性巨核细胞分化
Chloe A. L. Thompson-Peach [...] Daniel Thomas
2023年01月20日 2295 阅读
Abstract
Gut CD4 T cells are major targets of HIV-1 and are massively depleted early during infection. To better understand the mechanisms governing HIV-1-mediated CD4 T cell death, we developed the physiologically-relevant Lamina Propria Aggregate Culture (LPAC) model. The LPAC model is ideal for studying CD4 T cell death induced by clinically-relevant Transmitted/Founder (TF) HIV-1 strains and is also suitable for studying how enteric microbes and soluble factors (e.g., Type I Interferons) impact LP CD4 T cell death and function. Here, we detail the protocol to establish LP CD4 T cell infection using a process of spinoculation, the subsequent evaluation of infection levels using multicolor flow cytometry and the determination of overall LP CD4 T cell death using absolute LP CD4 T cell counts. We also describe the preparation of virus stocks of Transmitted/Founder (TF) HIV-1 infectious molecular clones that were successfully used in the LPAC model.
Keywords: Lamina Propria Aggregate Culture (固有层聚集培养)Background
CD4 T cell depletion is the hallmark pathogenic outcome of HIV-1 infection. However, the magnitude and kinetics of CD4 T cell depletion depends on the tissue compartment evaluated. The gastrointestinal tract is a major site of early HIV-1 replication and CD4 T cell death. At these early stages, the circulating HIV-1 strains primarily utilize CCR5 instead of CXCR4 as HIV co-receptors along with CD4. In fact, nearly all transmitted/founder (TF) HIV-1 strains utilize CCR5 (Keele et al., 2008; Salazar-Gonzalez et al., 2009). Unfortunately, CD4 T cells from peripheral blood mononuclear cells (PBMCs) and tonsil histocultures (the Human Lymphoid Aggregate Culture or HLAC model) are generally resistant to cell death following infection by CCR5-tropic HIV-1, likely due to the lack of CCR5 expression. Moreover, blood and tonsil CD4 T cells are mostly of the naïve and central memory subsets, which are less prevalent in the gastrointestinal tract. By contrast, gut CD4+ T cells are mainly of the effector memory subset and express high levels of CCR5. Gut CD4+ T cells also have higher representation of Th17 and Th22 subsets, which protect the integrity of the intestinal barrier, than that of blood or tonsil CD4+ T cells. Thus, peripheral blood and the HLAC model have limited utility in understanding TF HIV-1-mediated CD4+ T cell depletion in the gut. This prompted our group to adapt principles from the HLAC model (Doitsh et al., 2010; Doitsh et al., 2014) towards lamina propria mononuclear cells (LPMCs) (Steele et al., 2014).
The protocol for the isolation of LP mononuclear cells (LPMC) from human small intestinal tissue in the LPAC model is detailed in previous publications, and described in more detail in Procedure A (Howe et al., 2009; Dillon et al., 2010, 2012 and 2016; Steele et al., 2014; Harper et al., 2015; Yoder et al., 2017). In brief, LPMC are isolated from surgically resected small intestinal samples by collagenase-digestion after the removal of muscle, fat, mucus and epithelial layers. LPMC are cryopreserved at 5 x 106 to 10 x 106 LPMC per cryovial and have excellent overall viability on thawing (mean ± SEM: 87 ± 0.8% of total leucocytes (CD45+); N = 69, see Procedure B). Jejunum LPMCs consist mostly of CD3 T cells. On average, CD4 T cells constitute 65 ± 1.5% of viable LP leucocytes with smaller percentages of CD8 T cells, B cells and innate immune cells present (Figure 1). On average, 74 ± 3% (N = 30) of LP CD4 T cells express CCR5 (data not shown).
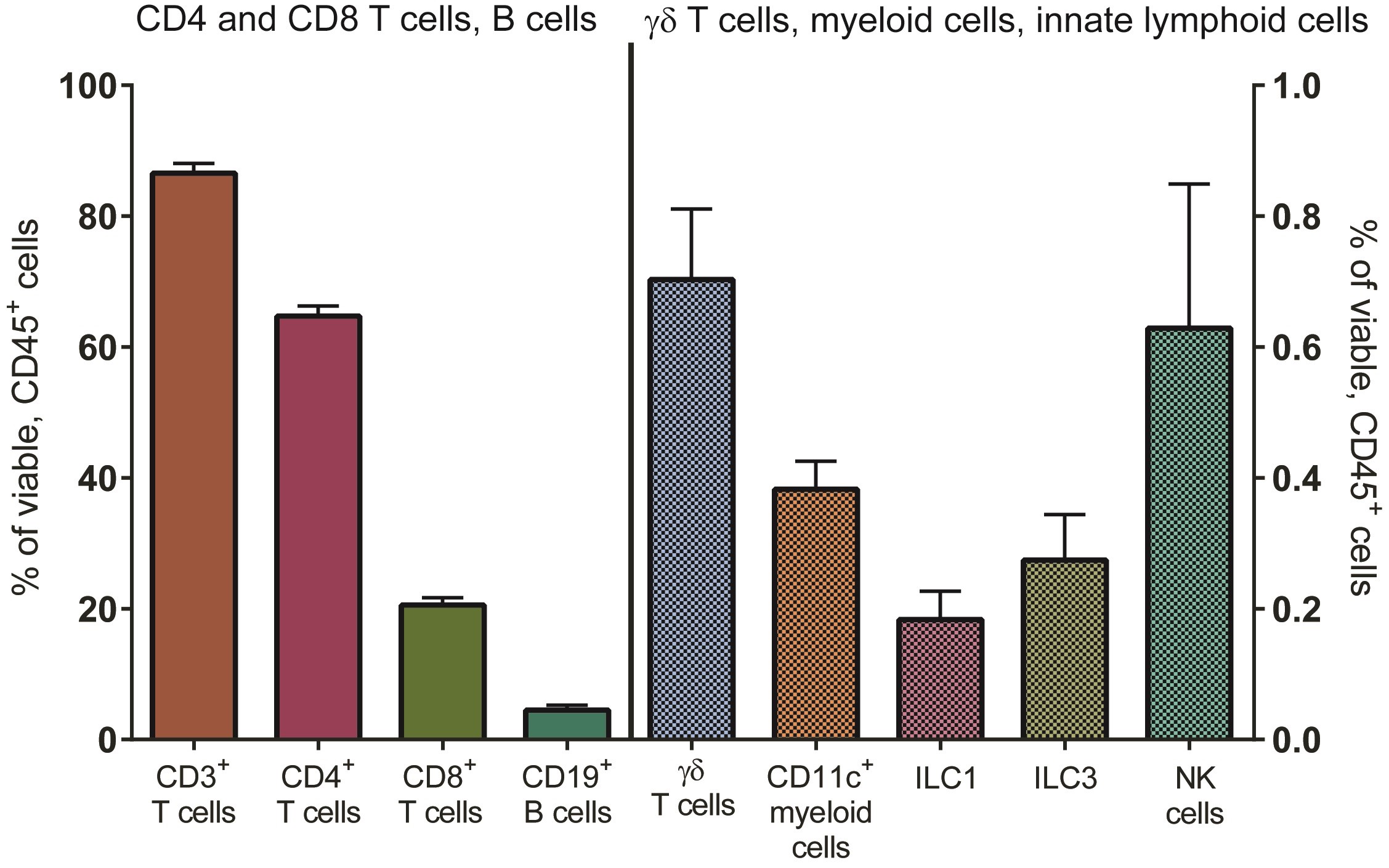
Figure 1. Distribution of immune cell populations in LPMC isolated from human jejunum tissue. Multi-color flow cytometry was used to determine frequencies of total CD3+ cells (n = 77), CD4+ cells (n = 75), CD8+ cells (n = 77), CD19+ B cells (n = 34) (left axis) and various innate immune cells including γδT cells (n = 45), CD11c+ myeloid cells (n = 50) and subsets of innate lymphoid cells (ILCs, n = 11) (right axis) within viable, leucocytes (CD45+).
Materials and Reagents
- 1.5 ml Eppendorf tube graduated (Fisher, catalog number: 05-408-137)
- 15 ml conical tubes (Thermo Fisher, catalog number: 339651)
- 50 ml conical tubes (Thermo Fisher Scientific, catalog number: 339652)
- 60 ml disposable syringe
- Vinyl Specimen Molds (Tissue Tek Cryomold, catalog number: 4566)
- Round-bottom Polystyrene tubes (“FACS tubes”) (Falcon, catalog number: 352052)
- Syringe-driven filter (0.45 μm) (Millipore, catalog number: SLHP033RS)
- Petri dish 100 mm x 20 mm (Fisher, catalog number: FB0875711Z)
- Polycarbonate thick wall ultracentrifugation tube (Beckman Coulter, catalog number: 355631)
- Cell strainer (70 μm) (Fisher, catalog number: 22363548)
- Mega Cassette System Tissue Tek (Sakura, catalog number: 4173)
- 96 V-bottom culture plates (Corning, catalog number: 3894)
- 96-well white tissue culture-treated sterile microplate (PerkinElmer, catalog number: 6005680)
- T175 culture flasks (Celltreat, catalog number: 229351)
- TZM-bl cells (AIDS Reagent Program, catalog number: 8129)
- TF infectious molecular clones:
- CH040 (AIDS Reagent Program, catalog number: 11740)
- CH058 (AIDS Reagent Program, catalog number: 11856)
- CH077 (AIDS Reagent Program, catalog number: 11742)
- CH470 (AIDS Reagent Program, catalog number: 12422)
- APC anti-human CD8 (Clone RPA-T8) (Tonbo Biosciences, catalog number: 20-0088)
- Bovine Serum Albumin (BSA) (Fisher Scientific, catalog number: BP1600-100)
- 293T cells (ATCC, catalog number: CRL-3216)
- RD-1 HIV-1 core antigen (Clone KC57) (Beckman Coulter, catalog number: 6604667)
- 0.25% Trypsin (Corning, catalog number: MT25053Cl)
- Collagenase D (Roche Diagnostics, catalog number 11088882001)
- DNase I (Sigma-Aldrich, catalog number: DN25-1G)
- Fetal Bovine Serum (FBS) (Life Technologies, catalog number: 26140079)
- HIV p24 antigen ELISA (Perkin Elmer, catalog number: NEK05001KT)
- Human AB Serum (Gemini Bioproducts, catalog number: 100-512)
- One ShotTM Stbl3 competent cells (ThermoFisher, catalog number: C737303)
- PE/Dazzle 594 anti-human CD3 (Clone UCHT1) (Biolegend, catalog number: 300450)
- 2 M CaCl2 (Sigma-Aldrich, catalog number: C1016)
- 10% Buffered Formalin Phosphate (Fisher Scientific, catalog number: SF100-4)
- 20% Sucrose solution in PBS
- 70% EtOH composed of Absolute EtOH200 proof (Fisher Scientific, catalog number: BP2818-4) diluted in Molecular Biology Grade Water (Hyclone, catalog number: SH30538.03)
- Ampicillin Sodium Salt (Fisher Scientific, catalog number: BP1760-5)
- BriteliteTM plus reagent (PerkinElmer, catalog number: 6066761)
- Cryotube Vial (Thermo Scientific, catalog number: 363401)
- Dextran Sulfate (Fisher Scientific, catalog number: BP1585-100)
- Dimethyl sulfoxide (DMSO) (Sigma, catalog number: D2650-5X10mL)
- DL-Dithiothreitol (DTT) (Sigma, catalog number: D9779-1G)
- Dulbecco’s Modified Eagle Medium (DMEM) (Corning, catalog number: MT10013CV.
- Dulbecco’s Phosphate Buffered Saline (DPBS) (GIBCO, catalog number: 14190-144)
- EDTA (Sigma-Aldrich, catalog number: E6511-100G)
- Fungizone (Amphotericin B) (Corning, catalog number: 30-003-CF)
- Hanks’ Balanced Salt Solution 1x (HBSS) (Corning, catalog number: 21-021-CM)
- HEPES (Sigma-Aldrich, catalog number: H0887-100mL)
- Kanamycin Sulfate (Fisher Scientific, catalog number: BP906-5)
- LB broth (Fisher scientific, catalog number: BP9723)
- Medium A (Fixation Medium) (Beckman Coulter, catalog number: GAS001S100)
- Medium B (Permeabilization Buffer) (Beckman Coulter, catalog number: GAS002S100)
- Paraformaldehyde (Fisher Scientific, catalog number: T353-500)
- Penicillin and streptomycin and L-glutamine (Gibco, catalog number: 10378-016)
- Penicillin-Streptomycin (Corning, catalog number: 30-002-Cl)
- Qiagen HiSpeed Plasmid Maxi Kit (Qiagen, catalog number: 12662)
- OTC Compound (Tissue Tek, catalog number: 4583)
- RNA Later Solution (Invitrogen, catalog number: AM7021)
- RPMI 1640 (RPMI) (Gibco, catalog number: 11875-093)
- SOC medium (Corning, catalog number: 46-003-CR)
- Trypan Blue (Corning, catalog number: 25-900-CI)
- Zombie Aqua Fixable Viability Dye (Biolegend, catalog number: 423102)
- Zosyn (piperacillin/tazobactium)(Novaplus, catalog number: 44567-801-10)
- KCl
- NaCl
- Na2PO4·7H2O
- Dextrose
- 1% Paraformaldehyde (see Recipes)
- Thaw Media (see Recipes)
- 2x HBS buffer (see recipes)
- Complete DMEM Media (see Recipes)
- Culture RPMI Media (see Recipes)
- Flow-staining buffer (see Recipes)
- Overnight holding media for jejunum tissue (see Recipes)
- Mucous removal media (see Recipes)
- Epithelium removal media (see Recipes)
- Collagenase Media for LPMC isolation (see Recipes)
- Preservative for isolated LPMCs (see Recipes)
- Complete RPMI medium for TF virus concentration (see Recipes)
- Modified epithelium removal media lacking BSA (see Recipes)
Equipment
- 37 °C water bath
- 37 °C incubator
- Centrifuge (Beckman Coulter, model: Allegra6R)
- Cool Cell LX Freezing Container (Biocision, catalog number: BCS-405)
- Germinator 500 (Surgical Equipment sterilizer) (Cell Point Scientific Inc., catalog number: 11931)
- Hemocytometer (Hausser Scientific, catalog number: 1492)
- Luminometer plate reader (PerkinElmer 2030 Multlabel Reader, model: VictorTM X5)
- Multi-channel pipette (Ranin 20-200 μl LTS, model: LM12-200)
- Multi-color Flow Cytometer (BD Biosciences, LSRII)
- Nutating Mixer Fixed Speed 120V (rotator) (Fisher Scientific, catalog number: 88-861-041)
- Orbital shaker (Forma Scientific, model: 4518)
- TC20 Automated Cell Counter (Bio-Rad, catalog number: 1450102)
- Stratacooler (Stratagene Agilent, catalog number: 401349)
- Surgical forceps (VWR, catalog number: 82027-388)
- Surgical scissors (VWR, catalog number: 82027-590)
Software
- FlowJo Software (Tree Star Inc.)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Dillon, S. M., Guo, K., Castleman, M. J., Santiago, M. L. and Wilson, C. C. (2020). Quantifying HIV-1-Mediated Gut CD4+ T Cell Death in the Lamina Propria Aggregate Culture (LPAC) Model. Bio-protocol 10(2): e3486. DOI: 10.21769/BioProtoc.3486.
分类
免疫学 > 免疫细胞功能 > 淋巴细胞
微生物学 > 微生物-宿主相互作用 > 病毒
细胞生物学 > 细胞分离和培养 > 细胞分离 > 流式细胞术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link