- EN - English
- CN - 中文
Semi-automated Model to Accurately Counting Sympathetic Nervous Fibers
交感神经纤维精确计数的半自动模型
发布: 2019年12月20日第9卷第24期 DOI: 10.21769/BioProtoc.3454 浏览次数: 4614
评审: Ehsan KheradpezhouhGeoffrey C. Y. LauAnonymous reviewer(s)

相关实验方案
基于 rAAV-α-Syn 与 α-Syn 预成纤维共同构建的帕金森病一体化小鼠模型
Santhosh Kumar Subramanya [...] Poonam Thakur
2025年12月05日 1367 阅读
Abstract
In recent years, the role of sympathetic nervous fibers in chronic inflammation has become increasingly evident. At the onset of inflammation, sympathetic activity is increased in the affected tissue. However, sympathetic fibers are largely absent from chronically inflamed tissues. Apparently, there is a very dynamic relationship between sympathetic innervation and the immune system in areas of inflammation, and hence a rapid and easy method for quantification of nerve fiber density of target organs is of great value to answer potential research questions. Sympathetic nerve ends lie in close proximity to immune cells in lymphoid tissues and lymphoid cells are equipped with catecholamine receptors. Catecholamines such as dopamine and adrenaline are secreted by sympathetic nervous fibers and can influence immune cell activity directly. Thereby the sympathetic nervous system immediately participates in the regulation of inflammation. Changes in innervation density could therefore indicate dysregulation of inflammatory processes. Currently, nervous fiber densities are either determined by tedious manual counting, which is not suitable for high throughput approaches, or by expensive automated processes relying on specialized software and high-end microscopy equipment. Usually, tyrosine hydroxylase (TH) is used as the marker for sympathetic fibers. In order to overcome the current quantification bottleneck with a cost-efficient alternative, an automated process was established and compared to the classic manual approach of counting TH-positive sympathetic fibers. Since TH is not exclusively expressed on sympathetic fibers, but also in a number of catecholamine-producing cells, a prerequisite for automated determination of fiber densities is to reliably distinguish between cells and fibers. Therefore, an additional stain using peripherin which is exclusively expressed in nervous fibers as a secondary marker was established. This new and simple method can be used as a high-throughput approach to reliably and quickly estimate sympathetic nervous system (SNS) nerve fiber density in target tissues.
Keywords: Peripheral nervous system (外周神经系统)Background
In order to provide less time-consuming alternatives to the tedious process of manually counting nervous fibers in tissues of interest and to stream line quantification and characterization of nervous fibers, automated and semi-automated processes have been developed and deployed as early as 1979. These processes require special equipment, such as array processors or specialized graphics ports and software, which is highly cost-intensive and often adapted to only one particular purpose (Frykman et al., 1979; Auer, 1994). To allow a more cost-efficient analysis of overall innervation in several target tissues, a semi-automated counting method was established by us. It is based upon several macros programmed for ImageJ using a basic fluorescence microscopy set-up. The working principle of the macros is demonstrated in Figure 1.
Usually, tyrosine hydroxylase (TH) is used as a marker of sympathetic fibers. TH catalyzes the conversion from L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA), which represents the rate-limiting step of catecholamine synthesis (Nagatsu et al., 1964; Hufton et al., 1995). TH is ubiquitously expressed in sympathetic nervous fibers as well as in a multitude of other cells throughout most mammalian tissues. Due to the fact that TH is not exclusively expressed in nervous fibers, we decided to introduce a counterstain to improve the distinction between TH-positive sympathetic fibers and TH-positive cells. The neurofilament peripherin is ubiquitously and exclusively expressed in nervous fibers and is therefore an excellent candidate for double stainings (Portier et al., 1983). We hypothesized, that with peripherin and TH co-staining, TH-positive fibers will be distinguished from TH-positive cells. Fibers will be discernible by the co-localization of peripherin and TH, while all other TH signals originate from TH-positive cells. If high-end technology required for automated counting processes is not available, fiber density is usually determined by manually counting visible TH-positive fibers in 17 high power fields (HPF), according to published methodology (Straub et al., 2008). However, this is a time-consuming and observer-dependent process. We present in this work a simple, high-throughput, automated screening method of sympathetic fiber density in tissues.
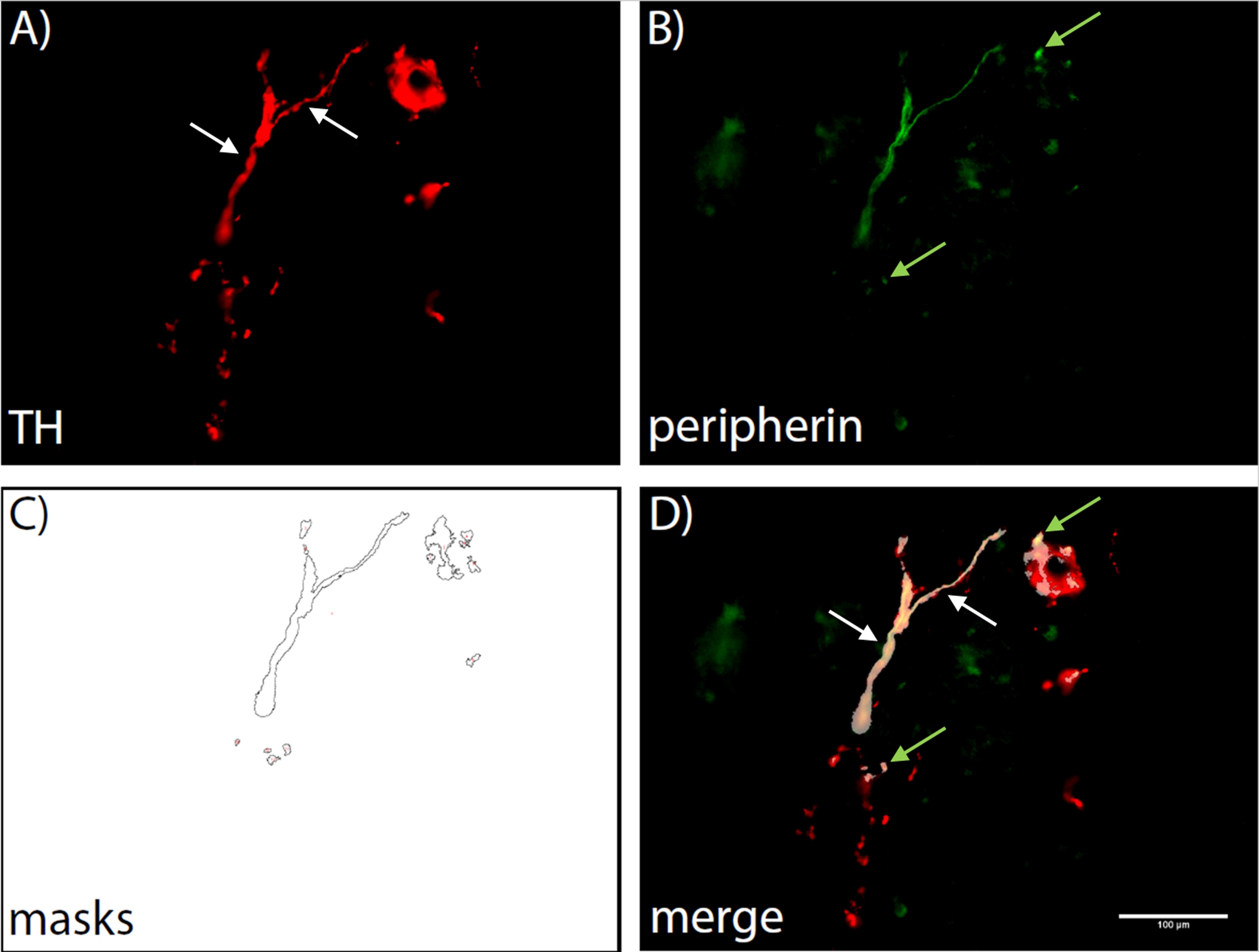
Figure 1. Demonstration of the automated counting process. A first macro creates a Region of interest (ROI) by automatically setting a threshold for TH positive areas (A). A second macro then lays the ROI over the corresponding peripherin stained image and creates masks wherever TH and peripherin staining are co-located (B, C). These masks are then counted and a list is displayed, showing the counts for each image that was analysed. A merge image is shown to illustrate the co-localization (D). White arrows indicate fibers that can be identified by eye, green arrows indicate those identified by co-localization only.
Materials and Reagents
- Pipette tips (Starlab, TipOne 1 ml, 200 µl, 10 µl, catalog numbers: S1111-6001, S1111-0000, S1111-3000)
- Microscopy cover glass (Engelbrecht, Cover Glass 24 mm x 60 mm, catalog number: K12460)
- Microtome blades (Feather, Disposable Microtome Blade, catalog number: C-35)
- Specimen molds (Sakura Finetek, Tissue-Tek Cryomold Intermediate 15 mm x 15 mm x 5 mm, catalog number: 4566)
- Microscopy slides (VWR, Adhesion slides SuperFrost Plus 75 mm x 25 mm, catalog number: 631-0108)
- PAP pen (Thermo Fisher, Super PAP Pen, catalog number: 008899)
- Erlenmayer flask (Brand, Erlenmayer flask 250 ml, catalog number: 92736)
- C57B/6J mice (Janvier Labs, C57B/6JRj, catalog number: C57B/6JRj)
- Goat Serum (NGS) (Agilent Dako, Goat Serum (Normal), Whole serum, 10 ml, catalog number: X090710-8), store at 4 °C
- Rabbit anti TH antibody (Merck, Anti-Tyrosine Hydroxylase Antibody, catalog number: AB152), store at -20 °C
- Chicken anti Peripherin antibody (Abcam, Anti-Peripherin antibody, catalog number: ab39374), store at -20 °C
- Goat anti Rabbit antibody (Invitrogen, Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 594, catalog number: A-11037), store at 4 °C
- Goat anti Chicken antibody (Abcam, Goat Anti-Chicken IgY H&L (Alexa Fluor® 488), catalog number: ab150169), store at 4 °C
- Embedding Medium (Sakura Finetek, Tissue-Tek® O.C.T.TM Compound, 12 x 125 ml, catalog number: 4583), store at room temperature (RT)
- Triton X (Sigma-Aldrich, Triton TM X-100, catalog number: T8787), store at RT
- Paraformaldehyde (PFA) (Sigma-Aldrich, Paraformaldehyde, reagent grade, crystalline, catalog number: P6148), store at 4 °C
- Hydrochloric acid (Sigma-Aldrich, Hydrochloric acid 36.5-38.0%, BioReagent, for molecular biology, catalog number: H1758), store at RT
- Sodium Hydroxide (Merck, Sodium hydroxide 1.000 l, catalog number: 1099130001), store at RT
- Prolong Gold (Invitrogen, ProLongTM Gold Antifade Mountant with DAPI, catalog number: P36931), store at 4 °C
- Phosphate buffered Saline (Gibco, PBS (10x), pH 7.4, catalog number: 70011044), store at RT
- Liquid nitrogen (Linde, CRYOSPEED MED, catalog number: 4130919)
- Deionized H2O
- 3% PFA (250 ml) (see Recipes)
- PBS 1x (see Recipes)
- Blocking solution (see Recipes)
- Washing solution (see Recipes)
- Secondary antibody diluent solution (see Recipes)
Equipment
- Cryostat (Leica, CM3000 Research Cryostat)
- Microscope (Zeiss, Axioskop 2 plus)
- Camera (Nikon, DSV Vi1)
- PC (Intel Core i3-7100U CPU, 2.40 GHz, 8.00 GB RAM, Windows 10 Enterprise, 64-bit, Intel HD Graphics 620)
- Magnetic stir heater (IKA, IKAMAG, catalog number: RET)
- -80 °C freezer (Thermo Scientific, Forma 906, catalog number: 906)
- Electronic scale (Sartorius, electronic precision scale, catalog number: 1465 MP8)
- Shaker (Peqlab, Mini Rocker-Shaker, catalog number: MR-1)
- Micro pipettes (Eppendorf, Research plus variable 0,1-1 ml, 20-200 µl, 2-20 µl, 0,5-10 µl, catalog numbers: 3123000063, 3123000055, 3123000039, 3123000020)
Software
- ImageJ (NIH, https://imagej.nih.gov/ij/)
- NIS-Elements (Nikon, https://www.nikon.com/)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Bleck, D., Erdene-Byambadoo, L., Brinks, R., Schneider, M. and Pongratz, G. (2019). Semi-automated Model to Accurately Counting Sympathetic Nervous Fibers. Bio-protocol 9(24): e3454. DOI: 10.21769/BioProtoc.3454.
分类
神经科学 > 行为神经科学 > 实验动物模型
神经科学 > 神经系统疾病 > 动物模型
神经科学 > 感觉和运动系统 > 动物模型
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link