- EN - English
- CN - 中文
Evoked Potential Recordings of Auditory Brainstem Activity in the Mouse: An Optimized Method for the Assessment of Hearing Function of Mice
小鼠听觉脑干活动的诱发电位记录:一种评估小鼠听觉功能的优化方法
发布: 2019年12月05日第9卷第23期 DOI: 10.21769/BioProtoc.3447 浏览次数: 6067
评审: Trevor Martin SmithPascal Fossat Anonymous reviewer(s)
Abstract
Hearing loss is a common sensory deficiency suffered by millions worldwide. It is a heterogeneous condition and genetics plays a critical role in its etiology. Gene variants can fundamentally alter hearing function, or predispose the auditory system towards loss of function resulting from other factors. In mouse studies of hearing loss and gene function, an evoked potential electrophysiological recording, the auditory brainstem response (ABR), is now considered the optimal way to screen large numbers of individuals, either with normal hearing sensitivity or with hearing impairment. Other routinely used methods to assess hearing function (such as acoustic startle responses, or otoacoustic emissions) do not allow assessment of the same broad spectrum of dysfunction nor readily allow the threshold sensitivity of the neural output of the cochlea to be assessed and are less ideal. An optimized recording system to rapidly and reproducibly record high-quality ABRs from mutant mice as part of a high-throughput phenotyping pipeline was developed. Click-evoked ABRs and ABRs evoked by pure-tone frequencies over a range of sound levels from 0 dB to 95 dB, sound pressure levels (SPL) are recorded. This takes approximately 15-20 min per mouse (with 5 tone frequencies), allowing a large number of mutant mice to be screened. This method has been used to measure ABRs on a high-throughput mutant mouse phenotyping pipeline and in laboratory tests to follow-up the hearing loss phenotypes identified on that pipeline.
Keywords: Hearing loss (听力损伤)Background
Hearing impairment is a widespread sensory deficiency suffered by millions worldwide, of all ages and gender. Effects sizes can range from mild to severe. It can be congenital or develop later in life, often in a frequency-dependent age-related progressive manner. Numerous and varied pathologies can contribute to or be responsible for hearing loss (Davis 1995; Fortnum et al., 2001). Environmental conditions, such as noise exposure or drug-toxicity, can also have profound effects on hearing ability. Genetics is known to play many crucial roles in the early development of hearing and in the maintenance of hearing function into old age, as well as in predisposing the auditory system towards pathology or protecting the system against pathological decline. With the development of molecular methods to target the function of specific genes in the mouse and the emergence of phenotyping programs to investigate the systemic roles of these genes, it became necessary to develop a sensitive, rapid, robust and reproducible method to screen hearing function in these mutant animals.
Measurement of the Preyer Reflex (an acoustic startle response) can be used to quickly test very high numbers of animals, but it can detect only severe hearing loss and may be affected by motor system deficiencies in mutant mice. Measurements of otoacoustic emissions can be performed quickly and non-invasively, but they do not detect as many pathological conditions as measures of the output of the cochlea as a whole. The Auditory Brainstem Response (ABR), an electrophysiological response incorporating auditory nerve and auditory brainstem activity, is considered the ideal method to assess hearing function across large numbers of mice.
Table 1. Comparison of methods of functional assessment of hearing in small mammals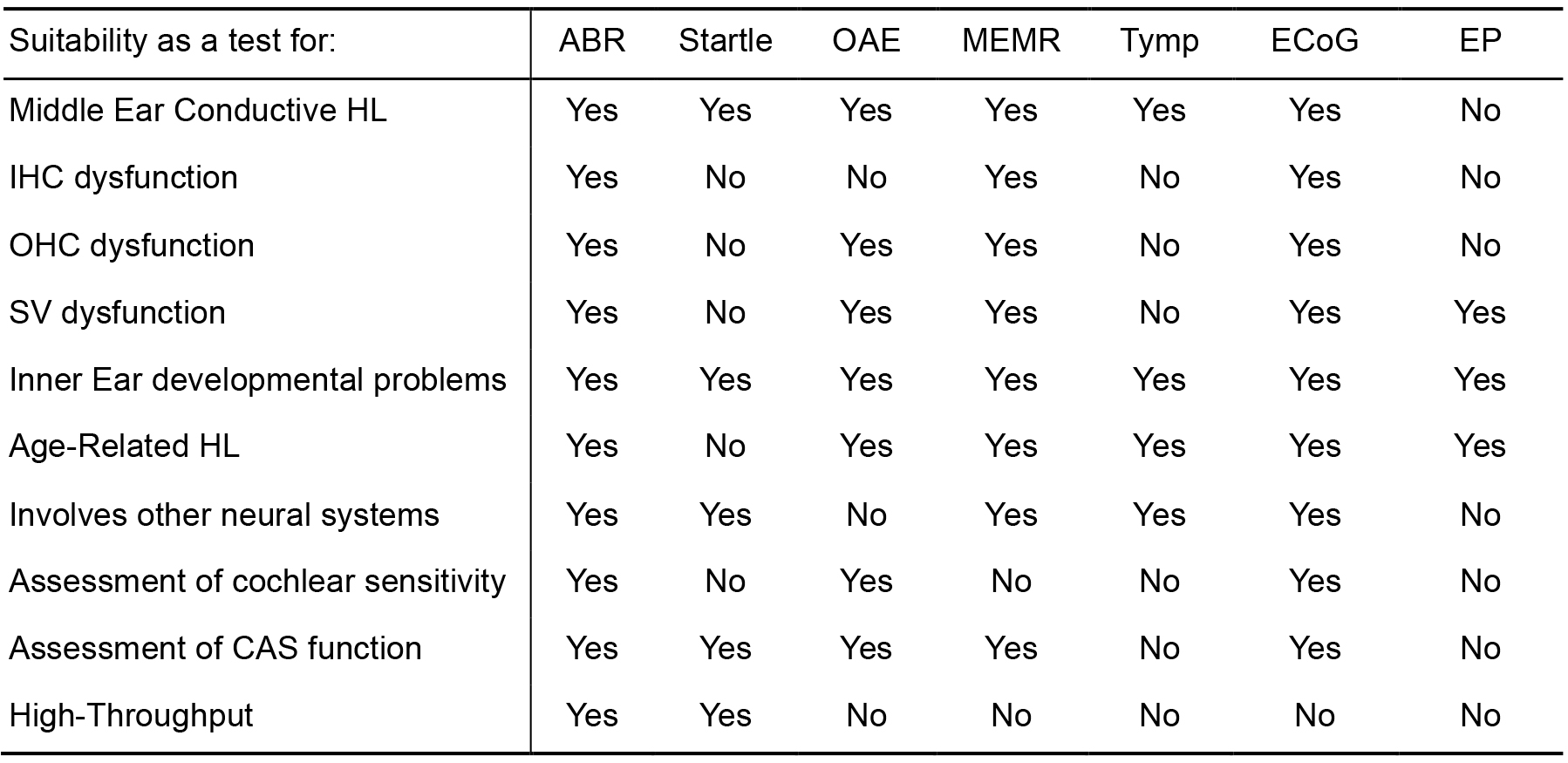
Methods considered are listed across Table 1: ABR, Auditory Brainstem Response. Startle, Startle Response (such as Preyer Reflex, Pre-Pulse Inhibition, etc.). OAE, Oto-Acoustic Emissions. MEMR, Middle Ear Muscle Reflex. Tymp, Tympanometry. ECoG, Electrocochleography. EP, Endocochlear Potential. The usefulness of a particular method to assess a particular function is indicated as a binary Yes/No choice. Yes, indicates that a test may give results to aid identification/diagnosis of a particular problem. No, indicates the test may not useful for this purpose. Middle Ear Conductive HL, is the test helpful to detect a conductive hearing loss, such as otitis media. IHC dysfunction, to detect Inner Hair Cell dysfunction. OHC dysfunction, to detect Outer Hair Cell dysfunction. SV dysfunction, to detect dysfunction of the Stria Vascularis. Inner Ear developmental problems, to detect early developmental issues affecting the inner ear. Age-Related HL, helpful to detect aspects of progressive hearing loss, if appropriate frequency stimuli are used. Involves other neural systems, can be influenced by dysfunction of other neural systems, such as descending inputs to the cochlea, motor systems, sensori-motor integration, etc. Assessment of cochlear sensitivity, to assess thresholds as an estimate of the sensitivity of cochlear function. Assessment of CAS function, to detect dysfunction within the Central Auditory System. High-Throughput, is the test amenable to high-throughput screening (e.g., simple and quick to set-up, short recording time, able to detect a broad spectrum of auditory dysfunction, etc.). The auditory brainstem response (ABR) is considered the best overall method to detect the widest spectrum of hearing impairment pathologies in a high-throughput screen where very large numbers (1000’s–10,000’s) of individual animals need to be assessed.
The method described here was adopted as the standard protocol used as a screen of hearing function by the International Mouse Phenotyping Consortium (IMPC, https://www.mousephenotype.org) at numerous research centers worldwide (summarized in Bowl et al., 2017). Detailed analyses of the data produced using this method at the Wellcome Sanger Institute have been published (White et al., 2013; Ingham et al., 2019). Whilst ABRs are commonly used to examine auditory function across a range of species, this method is optimized for rapid measurements in the mouse.
The ABR method, and our customized software, has been used in many secondary phenotyping studies to contribute to more detailed characterization of mutant phenotypes identified in the high-throughput screen (Ingham et al., 2019). The description presented here has been updated to reflect currently available digital signal processing technology. The method can easily be modified to perform evoked potential studies tailored towards more bespoke data collection and analysis requirements of individual studies (e.g., Chen et al., 2014; Buniello et al., 2016; Ingham et al., 2016) and can be used to assess more complex aspects of auditory function. For example, we can measure frequency tuning curves (Ebrahim et al., 2016), forward masking functions (Kuhn et al., 2011), and other measures of auditory temporal processing, based on assessment of ABR wave 1 amplitude (and latency). With training and experience, experimenters can use this system to generate high quality ABR recordings and produce reproducible measurements of threshold sensitivity, waveform shape and peak amplitude and latency, to give mean values with low associated variance helping to increase the statistical power of the method (e.g., Ingham et al., 2017).
Materials and Reagents
- Mice (Wildtype and Mutant mice, typically generated on a C57BL/6N genetic background, aged 13-14 weeks, were tested on the high-throughput screen [Ingham et al., 2019] although any strain of laboratory mouse aged, 14 days or older can easily be tested)
- Antisedan (Atipamezole Hydrochloride 5 mg/ml, Zoetis United States, Parsippany, New Jersery, USA)
- Ketaset (Ketamine Hydrochloride 100 mg/ml, Zoetis United States, Parsippany, New Jersery, USA)
- Rompun (Xylazine Hydrochloride 20 mg/ml; 2% w/v, Bayer plc, Berkshire, UK)
- Viscotears (Liquid Eye Gel 2 mg/g Carbomer, with cetrimide, Bausch & Lomb, Surrey UK)
- Ketamine/Xylazine anesthetic mix (made from Materials and Reagents #3 and #4; see Recipes)
- Atipamezole mix (made from Materials and Reagents #2; see Recipes)
Equipment
- Sound attenuating chamber (MAC1 or MAC2 Industrial Acoustics Company; or equivalent, with Field Noise Reduction of at least 37dB at frequencies of 2,000Hz and above, internal dimensions of approximately 584 x 406 x 356 mm [width x depth x height] or greater, incorporating radio-frequency shielding)
- Heating blanket (Homeothermic Blanket System; 50-7221F; Harvard Apparatus, Cambridge UK)
- Stimulus Generation and Signal Acquisition digital signal processing (DSP) equipment (RZ6-A-P1 Multi IO Processor, Tucker-Davis Technologies TDT, USA)
- Microphone and Signal Conditioner (Model 378C01, Model 480C02; PCB Piezoelectronics, NY, USA)
- Sound Transducer (Multifield speaker MF1; TDT, USA)
- BNC and other connector cables
- Subdermal Needle electrodes (SD51-426-1 NeuroDart, Spes Medica, Italy; 0.4mm diameter, 13mm length, with 1.5mm touchproof connector and 100cm cable length)
- Low-Impedance Recording Headstage/Preamplifier (RA4LI + RA4PA, or Medusa4Z; TDT, USA)
- Personal Computer (able to house a full-height PCI interface card)
- “Optibit” interface card (PO5E, TDT, USA)
- Digital Oscilloscope (TBS1000; Tektronix Berkshire UK)
Software
- RPvdsEx driver software (TDT, USA; www.tdt.com/support/downloads)
- ActiveX control software (TDT, USA; www.tdt.com/support/downloads)
- TeeChart AX software (Steema Software; Girona, Spain)
- Custom “Averager“ Software, written by Tim Folkard (Medical Research Council Institute of Hearing Research, Nottingham UK) and Neil Ingham, available on request from the author (neil.ingham@kcl.ac.uk)
- Custom “Traceview“ software, written by Tim Folkard (Medical Research Council Institute of Hearing Research, Nottingham UK) and Neil Ingham, available on request from the author (neil.ingham@kcl.ac.uk)
- ABR Analysis Software (written by Dr. B.N. Buran; https://github.com/bburan/abr) to facilitate peak and latency analyses
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Ingham, N. J. (2019). Evoked Potential Recordings of Auditory Brainstem Activity in the Mouse: An Optimized Method for the Assessment of Hearing Function of Mice. Bio-protocol 9(23): e3447. DOI: 10.21769/BioProtoc.3447.
分类
神经科学 > 感觉和运动系统 > 听力系统 > 听力损伤
生物物理学 > 电生理 > 听觉脑干反应
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link