- EN - English
- CN - 中文
An Optimized Method to Isolate Human Fibroblasts from Tissue for Ex Vivo Analysis
从组织中分离人成纤维细胞用于体外分析的优化方法
(*contributed equally to this work) 发布: 2019年12月05日第9卷第23期 DOI: 10.21769/BioProtoc.3440 浏览次数: 6336
评审: Gal HaimovichMarzia Di DonatoAnonymous reviewer(s)
Abstract
Despite their involvement in many physiological and pathological processes, fibroblasts remain a poorly-characterized cell type. Analysis of primary fibroblasts while maintaining their in vivo phenotype is challenging: standard methods for fibroblast isolation require cell culture in vitro, which is known to alter phenotypes. Previously-described protocols for the dissociation of primary tissues fail to extract sufficient numbers of fibroblasts, instead largely yielding immune cells. Here, we describe an optimized method for generating a fibroblast-enriched single-cell suspension from human tissues using combined mechanical and enzymatic dissociation. This allows analysis of ex vivo fibroblasts without the need for culture in vitro.
Keywords: Tissue disaggregation (组织解离 )Background
Fibroblasts are almost ubiquitous in human tissues, and the most common cell type in the stroma of a number of solid tumours, where they are referred to as cancer-associated fibroblasts (CAFs) (Ishii et al., 2016; Kalluri and Zeisberg, 2006; Rupp et al., 2014; Servais and Erez, 2013). CAFs are associated with multiple hallmarks of malignancy (Ishii et al., 2016; Tao et al., 2017) and correlate with poor prognosis in multiple solid tumours (Hanley et al., 2018).
Given these tumour-promoting effects, and their genetic stability relative to cancer cells (Ishii et al., 2016), it is unsurprising that fibroblasts are an attractive therapeutic target. However, clinical trials targeting CAFs have so far yielded disappointing results (Hofheinz et al., 2003; Narra et al., 2007). This may, in part, be due to variation within the fibroblast population: these cells are known to be heterogeneous in both normal and disease states (Desmoulière et al., 2004; Sugimoto et al., 2006; Anderberg and Pietras, 2009; Servais and Erez, 2013; Witowski et al., 2015; Kalluri, 2016; Mellone et al., 2017). However, this heterogeneity remains poorly-characterised and it is not yet clear how many subtypes are present within a given tissue or tumour type, or the nature of functional differences between groups (Herrera et al., 2013; Servais and Erez, 2013; Ishii et al., 2016).
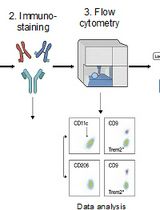
Characterising heterogeneity of cell populations within human tissues often requires analysis at a single-cell level. Single-cell RNA sequencing is a valuable platform for characterising multicellular ecosystems. However, fibroblasts are embedded within extracellular matrix and are particularly difficult to isolate: this has led to under-representation in, for example, single-cell RNA sequencing datasets (Lambrechts et al., 2018). Unlike many murine models, there is no standardized disaggregation protocol for human solid tissues. A number of different immune cell populations have been successfully isolated and analyzed directly from tissues (Holt et al., 1986; Perrot et al., 2007; Grange et al., 2011; Quatromoni et al., 2015; Ganesan et al., 2017). However, epithelial and non-immune stromal cells are usually cultured in vitro prior to analysis (Lurton et al., 1999; Koumas et al., 2003; Comhair et al., 2012; Barkauskas et al., 2013; Mackay et al., 2013). Culture in vitro has been shown to change fibroblast phenotypes (Öhlund et al., 2017; Waise et al., 2019); thus, how and whether the functional differences described in vitro are maintained in vivo is not yet known (Lurton et al., 1999).
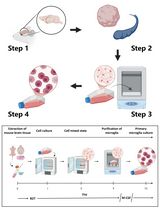
Here, we describe an optimized protocol for the isolation of fibroblasts from primary human tissues, allowing immediate analysis without the need for culture in vitro. In brief, primary samples undergo mechanical and enzymatic dissociation, followed by incubation with TrypLE and red cell lysis buffer (to disrupt intercellular adhesions and remove red blood cells, respectively). Use of this approach yields a single-cell suspension consisting of multiple cell types, with approximately a 4-fold greater proportion of fibroblasts compared to other disaggregation strategies (Waise et al., 2019). We describe use of this protocol for the ex vivo analysis of fibroblasts in both normal and disease states using single-cell RNA sequencing, and highlight alternative downstream applications. In addition, this approach may have applications in the analysis of other cell types (e.g., epithelial cells).
Materials and Reagents
- 5 ml syringes (BD Plastipak, catalog number: 307731)
- 10 ml syringes (BD Plastipak, catalog number: 307736)
- Pasteur pipette (Scientific Laboratory Supplies, catalog number: PIP4210)
- 50 ml Falcon tube (Sarstedt, Brand, model/catalog number: 114 x 28 mm, 62.547.004)
- 15 ml Falcon tube (Sarstedt, Brand, model/catalog number: 120 x 17 mm, 62.554.002)
- Sterile scalpel #21 blade (Swann-Morton, catalog number: 0507)
- Polystyrene cell culture dish (Sarstedt, catalog number: 83.3902)
- Syringe filtration unit Filtropur S 0.2 (Sarstedt, catalog number: 83.1826.001)
- Scissors
- Collagenase P from Clostridium histolyticum (Merck, Roche, catalog number: 11213857001). Reconstitute in PBS to 150 U/ml, store 100 μl aliquots at -20 °C
- TrypLE Express Enzyme (no phenol red; Thermo Fisher, catalog number: 12604013). Store at room temperature protected from light for up to 2 years
- Fetal bovine serum (Biosera, catalog number: FB-1001/500). Store at -20 °C for up to 60 months
- Deoxyribonuclease I from bovine pancreas (Merck, Sigma-Aldrich, catalog number: D4263). Reconstitute in 1 ml PBS (2000 U/ml), store 40 μl aliquots at -20 °C
- Dulbecco’s Modified Eagle Medium (Merck, Sigma-Aldrich, catalog number: D5671-500ML). Store at 4 °C
- L-glutamine (Merck, Sigma-Aldrich, catalog number: G7513-100ML). Store at -20 °C for up to 2 years
- Penicillin-streptomycin (Merck, Sigma-Aldrich, catalog number: P4333-100ML). Store at -20 °C for up to 2 years
- Phosphate-buffered saline (PBS)
- Amphotericin B (250 μg/ml; Gibco, catalog number: 15290-018). Store at -20 °C for 1 year
- Sterile double-distilled H2O
- Red blood cell lysis buffer (10x; BioLegend, catalog number: 420301). Store at 4 °C
- DNase stock solutions
- “Complete” DMEM (see Recipes)
- “Empty” DMEM (see Recipes)
Equipment
- Orbital shaker-incubator (e.g., Grant-bio Orbital Shaker-Incubator ES-20)
- EASYStrainer 40 μm (Grener Bio-One, catalog number: 542040)
- Centrifuge
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Waise, S., Parker, R., Rose-Zerilli, M. J. J., Layfield, D. M., Wood, O., West, J., Ottensmeier, C. H., Thomas, G. J. and Hanley, C. J. (2019). An Optimized Method to Isolate Human Fibroblasts from Tissue for Ex Vivo Analysis. Bio-protocol 9(23): e3440. DOI: 10.21769/BioProtoc.3440.
分类
癌症生物学 > 通用技术 > 肿瘤微环境
细胞生物学 > 细胞分离和培养 > 细胞分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link