- EN - English
- CN - 中文
Mapping the Mechanome–A Protocol for Simultaneous Live Imaging and Quantitative Analysis of Cell Mechanoadaptation and Ingression
力学组学映射—一种用于同时进行实时成像和细胞力学适应和侵入定量分析的方案
(*contributed equally to this work) 发布: 2019年12月05日第9卷第23期 DOI: 10.21769/BioProtoc.3439 浏览次数: 6387
评审: Ralph Thomas BoettcherSurabhi SonamAnonymous reviewer(s)
Abstract
Mechanomics, the mechanics equivalent of genomics, is a burgeoning field studying mechanical modulation of stem cell behavior and lineage commitment. Analogous to mechanical testing of a living material as it adapts and evolves, mapping of the mechanome necessitates the development of new protocols to assess changes in structure and function in live stem cells as they adapt and differentiate. Previous techniques have relied on imaging of cellular structures in fixed cells and/or live cell imaging of single cells with separate studies of changes in mechanical and biological properties. Here we present two complementary protocols to study mechanobiology and mechanoadaptation of live stem cells in adherent and motile contexts. First, we developed and tested live imaging protocols for simultaneous visualization and tracking of actin and tubulin mechanoadaptation as well as shape and volume of cells and their nuclei in adherent model embryonic murine mesenchymal stem cells (C3H/10T1/2) and in a neuroblastoma cell line. Then we applied the protocol to enable quantitative study of primary human mesenchymal stem cells in a motile state, e.g., ingression in a three-dimensional, in vitro cell culture model. Together, these protocols enable study of emergent structural mechanoadaptation of the cell's own cytoskeletal machinery while tracking lineage commitment using phenotypic (quantitative morphology measures) and genotypic (e.g., reverse transcription Polymerase Chain Reaction, rtPCR) methods. These tools are expected to facilitate the mapping of the mechanome and incipient mechanistic understanding of stem cell mechanobiology, from the cellular to the tissue and organ length scales.
Keywords: Live cell imaging (活细胞成像 )Background
Collectively referred to as mechanomics, the study of how mechanical cues modulate stem cell behavior has grown rapidly in the past decade (Figure 1). Experimental and computational mechanomics studies have demonstrated the profound influence of mechanical environment on cell motility (Knothe Tate et al., 2008; Blanchoin et al., 2014; Aubry et al., 2015; Knothe Tate et al., 2016; De Pascalis and Etienne-Manneville, 2017; Ladoux and Mege, 2017), stem cell niche quiescence (Yu et al., 2017; Ni et al, 2019) and lineage commitment (Anderson et al., 2006; Anderson and Knothe Tate 2007a and 2007b; Knothe Tate et al.,2008; McBride and Knothe Tate, 2008; McBride et al., 2008; Song et al., 2010; 2012 and 2013; Zimmermann et al., 2011; Chang and Knothe Tate, 2011; Earls et al., 2013; Heo et al., 2015; Nimmo et al., 2015; Steward and Kelly, 2015; Le et al., 2016; Stumpf et al.,2017; Galarza et al., 2018). Mechanical testing of live cells, as they adapt and differentiate, necessitates the development and testing of mechanobiological tools incorporating imaging and quantitative measures of shape, volume, and architecture changes (e.g., of cells, as well as their nuclei and cytoskeletons) for controlled loading scenarios, with relevant spatial and temporal resolution (Anderson et al., 2006; Anderson and Knothe Tate 2007a and 2007b; McBride and Knothe Tate, 2008; McBride et al., 2008; Song et al., 2010; 2012 and 2013; Zimmermann and Knothe Tate, 2011; Chang and Knothe Tate, 2011).
During development and postnatal healing, the mechanical cues to which stem cells are subjected exert a profound role in the cells' capacity to self-assemble structure (Figure 1), which manifests in several ways. The cell's internal structures such as the cytoskeleton self-assemble and adapt in response to the mechanical environment. Cells themselves self-assemble into multicellular constructs. Cells also create tissue architectures through transcription, assembly and post-translational modification of extracellular matrix (Knothe Tate et al., 2016).
Prior to lineage commitment, stem cells act as sensors and actuators, transducing mechanical signals from the local environment to the nucleus, where gene transcription is up- and down-regulated, resulting in genesis of tissue templates that grow and mature over time (Knothe Tate et al., 2008; Knothe Tate et al., 2016; Ng et al., 2017). Embryonic mesenchymal stem cells exhibit intrinsic sensitivity to mechanical stress; these cells change their baseline gene expression in response to subtle mechanical cues three orders of magnitude smaller than those to which e.g., trigger changes in baseline gene expression of adult chondrocytes of the knee joint (McBride and Knothe Tate, 2008; McBride et al., 2008; Song et al., 2012). In addition, conditionally knocking out specific constituents of stem cells’ mechanosensing apparatus causes stem cells to lose their capacity to self-assemble structure and hence tissues (Knothe Tate et al., 2010).
The geometric arrangement of cells in space and time results in patterning of the organism’s template during prenatal development as well as the injured and/or missing tissue template during postnatal healing. The process is gated by cell motility and adherence (with adherence defined by the lack of capacity to move) (Knothe Tate et al., 2008; Evans et al., 2013). Until recently, mechanistic and dynamic study of unfolding cell fate and tissue template genesis has been stymied by the lack of methods to study these processes in situ, in live multicellular constructs. This protocol presents methods to study cell motility in model constructs as well as mechanoadaptation of the cytoskeleton within the cell itself. Pilot study data demonstrates the utility of the methods.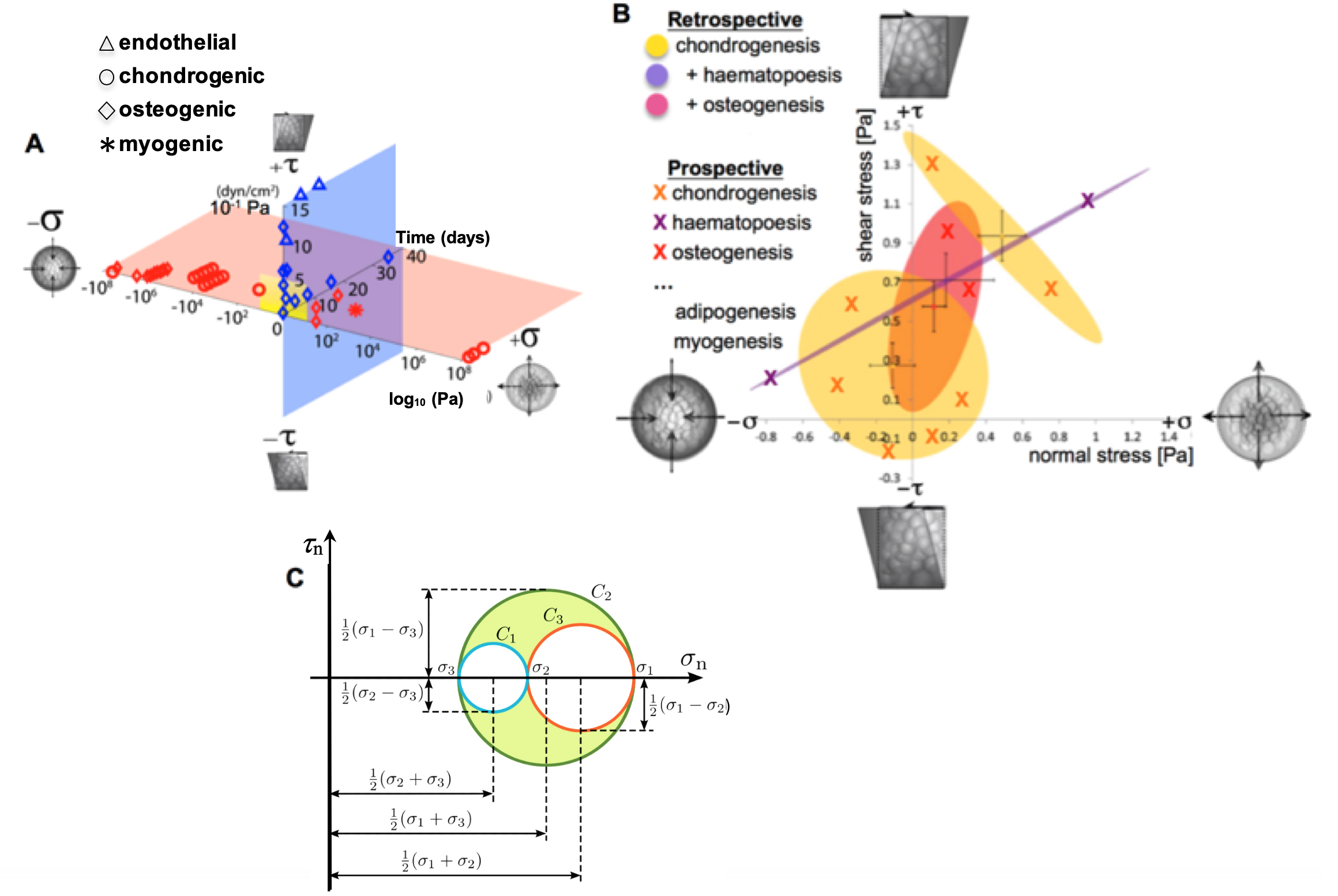
Figure 1. Mapping the mechanome at the interface of genomics and mechanics of materials. Cell behavior is modulated by biophysical cues including volume (dilatational–compression and tension) and shape changing (deviatoric–shear) stresses. A. Mechanical loading throughout life literally shapes the structure and function of cells. Cells sense mechano-chemical stimuli and prototype tissue templates via up and downregulation of structural protein transcription, secretion into the extracellular matrix, and post-translational modification. This figure depicts characteristic magnitudes and time domains of mechanical signals applied in studies of multipotent cell differentiation, with nascent lineage commitment depicted by the shape of the data points. Tissue genesis and adaptation represents a continuum in space and time, over the life cycle of the individual organism, from development of the body template in utero (depicted 11.5 days after fertilization at the first stages of skeletogenesis in the mouse) and in the adult human. Image after (Ng et al., 2017) as adapted from (Anderson and Knothe Tate 2017b; Song et al., 2013) and used with permission. B. Using paired, live imaging and computational modeling approaches from our consortium, previous published studies mapped the mechanome retrospectively, depicting real stress state data from the coupled computational modeling and imaging studies in relation to 95% confidence intervals (shaded ovals) of nascent lineage commitment data (yellow, pink, violet), measured using rtPCR (Song et al., 2010; 2012 and 2013; Ng et al., 2017; Anderson and Knothe Tate, 2017b). Current and future approaches implement stress states from non-overlapping fates to prospectively test fate guidance through delivery of mechanical cues (B, e.g., X indicates chondrogenesis, in yellow; +haematapoesis, in purple; +osteogenesis in fuschia) that induce volume (dilatational) and shape (deviatoric) changing stresses at cell surfaces. This is akin to conducting a mechanical test on a stem cell during the process of lineage commitment. Adapted from original with permission (Song et al., 2013). C. An equivalent classical mechanics Mohr’s Circle diagram graphically represents the stress tensor obtained by performing a stress analysis on a material body such as a cell. Classical continuum mechanics’ principal limitation in biology is that it cannot adequately address biological behavior of live materials that evolve over time, e.g., via motility, mechanoadaptation, and differentiation (Knothe Tate et al., 2011 and 2016).
Overview of Approach
Live imaging methods were first developed for simultaneous tracking of actin and tubulin transcription and dynamic cytoskeletal mechanoadaptation, as well as changes in nucleus shape and volume, in adherent cells (Figure 2). This model system was designed to enable study of emergent structural mechanoadaptation of the cell's own cytoskeletal machinery while measuring emergent lineage commitment using phenotypic (morphology) and genotypic (rtPCR) methods. The C3H/10T1/2 cell line was used for the intracellular mechanoadaptation studies to insure cell-to-cell standardization. Derived from the mesenchyme, the C3H/10T1/2 murine pluripotent embryonic cells do not show phenotypic drift and are capable of differentiating along osteogenic, chrondogenic, adipogenic, smooth muscle, and endothelial lineage paths. This established cell line was used previously by our research consortium in a series of mechanoadaptation studies (McBride and Knothe Tate, 2008; McBride et al., 2008; Song et al., 2010; 2012 and 2013; Chang and Knothe Tate, 2011; Zimmermann and Knothe Tate, 2011). In addition, we tested the protocol on the rat neuroblastoma B35 cell line (Tpm3.1 neuroblastoma), an immortalized and well characterized cell line that displays prominent actin stress fibers (Bryce et al., 2003). Finally, use of these two different cell lines enabled us to optimize the protocol, accurately and efficiently, for use in primary cell cultures as well as cell lines.
Thereafter, an in vitro model was developed for dynamic, live imaging and tracking of stem cells ingressing from a seeded surface to the interior of an idealized tissue template, mimicking an epithelial to mesenchymal transition (EMT) (Li et al. 2014) in a model tissue template (Anlage) during development or postnatal healing (Figure 2). To maximize clinical relevance, we tested the tissue ingression protocol using primary mesenchymal stem cells isolated from adult human periosteum (periosteum-derived stem cells, PDCs; Human ethics protocol approved) (Chang and Knothe Tate, 2011; Zimmermann and Knothe Tate, 2011) as well as bone marrow derived stem cells (BMSCs, adult human) from a commercial vendor.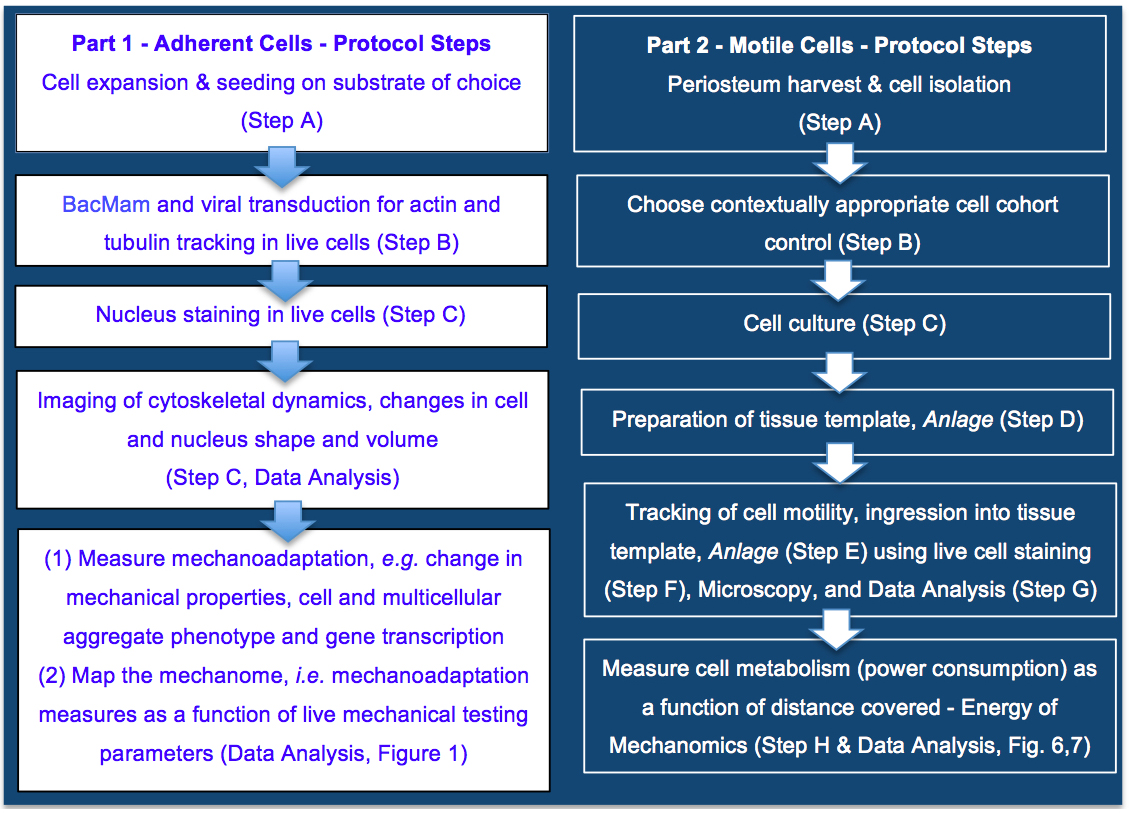
Figure 2. Protocol for mapping the mechanome at the interface of genomics and mechanics of materials, on cells adherent to tissue template scaffolds or functionalized coverslips (Part 1) or ingressing into idealized tissue templates (Part 2). Protocols steps are depicted in context of the aim to map the mechanome throughout cell and tissue genesis, in developmental and postnatal healing contexts where cells exist in adherent or motile states. Part 1. Mapping the mechanome by tracking changes in cell/nucleus shape & volume and actin and tubulin as a function of gene transcription and mechanoadaptation, similar to a mechanical test of a living material as it evolves and adapts. Part 2. Energy of Mechanomics by live imaging and tracking of stem cell ingression from a seeded surface to the interior of an idealized tissue template as a function of cell metabolism, enabling assessment of the energy of mechanomics. The approach measures metabolic energy expended by the cell over time (power) as a function of distance covered by the motile cell.
Materials and Reagents
Note: +Examples of items available from multiple vendors.
- Eppendorf tube
- T-75 culture flasks (+Corning, catalog number: 430641U)
- 25-mm plain glass coverslip
- 6-well plate (+Corning, catalog number: CLS 3516)
- 24-well plate (+Corning, catalog number: CLS 3524)
- Petri dish (+Corning, catalog number: CLS 430166)
- Serological pipette (5, 10, 25 ml) (+Corning, catalog numbers: CLS 4487, 4488, 4489)
- Specimen jars (+Sardstedt, catalog number: SAR75.9922.534)
- FalconTM cell strainer (+Fischer Scientific)
- Cryovials/cryogenic vials/cryotubes (+Fisher Scientific, catalog number: 12-565-169N)
- Glass bottom dish (Cellvis, catalog number: D35-10-0-N)
- Ovine femora (fresh from butcher)
- Cells tested in the current protocol
- C3H/10T1/2 murine embryonic mesenchymal progenitor cell line (CCL-226, ATCC)
- Rat neuroblastoma B35 cell line (Tpm3.1 neuroblastoma) cell line (after Bryce et al., 2003; Bach et al., 2010; Jalilian et al., 2015)
- PDSCs, Primary Periosteum Derived mesenchymal Stem Cells (after Knothe Tate, 2011; Evans et al., 2013; Chang et al., 2014)
- BMSCs, Bone marrow derived Mesenchymal Stem Cells (Lonza, catalog number: PT 2501, lot no. 0000636886)
- Fetal bovine serum (+Gibco, catalog number: 26400044)
- L-glutamine (Sigma, catalog number: G7513-100ML)
- Penicillin/streptomycin (+Sigma, catalog number: P4333-100ML)
- DMSO (+Sigma, catalog number: 472301-100ML)
- Trypsin-EDTA (+Sigma, catalog number: T4049-500ml)
- Cellular LightsTM actin-GFP (CellLight® Actin-RFP, BacMam 2.0, Molecular Probes/Life Technologies, catalog number: C10583)
- CellLight® Tubulin-GFP, BacMam 2.0 (Molecular Probes/Life Technologies, catalog number: C10613)
- PBS (Phosphate-buffered saline) (UNSW Upper campus store, catalog number: UCS-BIO-0040)
- Hanks buffer (Hoechst 33342, Trihydrochloride, Trihydrate) (Thermo Fisher Scientific, catalog number: H3570)
- Collagenase II (Gibco, catalog number: 17101015)
- α-minimal essential medium (α-MEM) with GlutaMAX (+Life Technologies, catalog number: 32561037)
- Antibiotic-antimycotic (AMAB) (Gibco, catalog number: 15240062)
- 0.4% (wt/vol) trypan blue salt solution (+Sigma, catalog number: T8154-100 ML)
- Liquid nitrogen
- Matrigel®, phenol red free (Corning, catalog number: 356237)
- Calcein-AM (+Sigma-Aldrich, catalog number: 17783-1MG)
- MTT (Thiazolyl Blue Tetrazolium Bromide) Metabolic Assay (Sigma-Aldrich, catalog number: M5655-100MG)
Equipment
- Centrifuge
- Humidified incubator
- Hemocytometer
- Perfusion and imaging chamber (+ProFlow Chamber, Warner Apparatus, PFC-1)
- Leica SP8 DLS confocal laser scanning microscope or other fluorescent microscopes with tunable excitation and emission wavelengths
- Plate reader (Perkin Elmer Victor 3, 570 nm filter)
Software
- Statistics and graphical analysis software (+Prism 7 software, GraphPad)
- Image analysis software (+ImageJ, Reference 36)
Part 1–Tracking change in cell/nucleus shape & volume, cytoskeletal transcription & mechanoadaptation
Here we implemented multiplexed imaging and tracking of changes in the nucleus, actin filaments and microtubules, in live cells and at high resolution using a viral gene delivery system to insert the desired gene into the host's genome (Shoji et al., 1997; Airenne et al., 2003; Ho et al., 2005; Kost et al., 2005; Salminen et al., 2005). The main advantage of the technique is that both actin and tubulin monomers are fluorescently tagged through the cellular transcriptional system and then individual monomers get incorporated into cytoskeletal structures by cells. This method offers rapid, safe, easy and convenient steps for delivery of desired gene to different mammalian cell lines but may not be efficient in certain cell types, including primary ovine Periosteum Derived Stem Cells, PDSCs (Chang and McBride, MechBio Team unpublished data). Here we optimized the method for the aforementioned C3H/10T1/2 murine embryonic stem cell line and the immortalized Tpm3.1 neuroblastoma cell lines. These two cell lines were chosen specifically because the transduction of two different cell types let us measure the amount of transduction efficiency and protein expression in a quantitative and robust way.
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Putra, V. D. L., Jalilian, I., Campbell, M., Poole, K., Whan, R., Tomasetig, F. and Knothe Tate, M. L. (2019). Mapping the Mechanome–A Protocol for Simultaneous Live Imaging and Quantitative Analysis of Cell Mechanoadaptation and Ingression. Bio-protocol 9(23): e3439. DOI: 10.21769/BioProtoc.3439.
分类
系统生物学 > 机械力组学 > 机械适应性
干细胞 > 多能干细胞 > 细胞分化
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link