- EN - English
- CN - 中文
Gallyas Silver Impregnation of Myelinated Nerve Fibers
有髓神经纤维的Gallyas银浸染法
发布: 2019年11月20日第9卷第22期 DOI: 10.21769/BioProtoc.3436 浏览次数: 4888
评审: Oneil G. BhalalaCarey Y. L. HuhAnonymous reviewer(s)
Abstract
In the nervous system of vertebrates, nerve impulse propagation is accelerated by the ensheathment of neuronal axons with myelin. Myelin sheaths are molecularly specialized, lipid-rich plasma membrane extensions of Schwann cells in the peripheral nervous system and oligodendrocytes in the central nervous system (CNS). To visualize myelinated nerve fibers and to allow for the morphological analyses of myelin in the brain and the spinal cord, an efficient method for silver impregnation of myelin has originally been developed by Ferenc Gallyas in 1979, referred to as Gallyas silver impregnation. Gallyas’ method is based on the agyrophilic characteristic of myelin to form and bind silver particles, while this process is suppressed in tissues other than myelin. The silver particles are finally enhanced in a developing step (“physical developer”). The main advantage of this method is that it efficiently visualizes both large myelinated fiber tracts and individual myelinated axons. Here we provide our laboratory protocol that is suitable for paraffin embedded sections and the use of light microscopy based on Gallyas’ original protocol and subsequent modifications by Pistorio and colleagues.
Keywords: Myelin (髓鞘)Background
Myelin is the multilayered, molecularly specialized plasma membrane of oligodendrocytes in the CNS or Schwann cells in the peripheral nervous system that accelerates nerve conduction by ensheathing axons (for comprehensive reviews: Hildebrand et al., 1993; Kidd et al., 2013; Nave and Werner, 2014; Monk et al., 2015; Simons and Nave, 2015). The myelination of axons provides electrical insulation and thus facilitates saltatory impulse propagation in a highly efficient manner (Tasaki, 1939; Hartline and Colman, 2007). In addition to insulation, myelinating cells may provide trophic support for myelinated axons (Lappe-Siefke et al., 2003; Nave, 2010; Funfschilling et al., 2012; Joseph et al., 2019).
Electron microscopy reveals morphologically distinguishable subcompartments of myelin. The compacted myelin comprises alternating electron-dense and electron-lucent layers while the adaxonal myelin layer and paranodal myelin loops remain non-compacted. At the light microscopic level, large myelinated tracts (i.e., the white matter including the corpus callosum) are clearly distinguishable from the grey matter, in which the majority of axons is non-myelinated. However, even largely unmyelinated CNS regions including the cortex comprise individual myelinated axons that appear as fine fibers if visualized by specific staining.
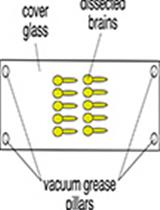
Ferenc Gallyas described in 1979 a method to specifically visualize myelin in the brain (Gallyas, 1979). A fundamental step is the treatment of the CNS tissue with pyridine and acetic anhydride, which during the subsequent silver impregnation prevents the absorption of silver ions by tissues other than myelin. Another critical step is the physical developer, which contains silver nitrate and tungstosilicic acid, which, once added, reduces silver ions to elemental silver. In 2005, Pistorio and colleagues described a modified version of this method by Gallyas (Pistorio et al., 2006). Here, we provide our lab protocol based on these previous versions. The major difference is the use of a microwave to decrease the staining time as heat causes the molecules to diffuse rapidly across tissues. Other differences include a slight modification in the recipe of the physical developer and the absence of a de-staining step involving potassium ferricyanide. In recent years, silver impregnation of myelin has been mainly used to assess myelinated tracts in mouse mutants (de Monasterio-Schrader et al., 2012; Werner et al., 2013; Patzig et al., 2016; Erwig et al., 2019; Joseph et al., 2019), yet the method can be applied to various species and scientific questions. As we describe the silver impregnation of paraffinated brain sections, we included the paraffin embedding and the de-paraffinization procedure.
Materials and Reagents
- Cover slips (Thermo Scientific, Menzel, catalog number: 1011880500)
- Microscope glass slides (R. Langenbrinck, catalog number: 03-0003)
- Paraffin
- 50 ml Falcon tube
- Mice (postnatal as well as adult brains of any mouse strain are suitable for Gallyas staining)
- 2-propanol (Roth, catalog number: 6752.4)
- Acetic acid (Merck Millipore, catalog number: 1000631000)
- Acetic anhydride (Acros organics, catalog number: 149490010)
- Ammonium nitrate (Merck Millipore, catalog number: 1011880500)
- Ethanol (Roth, catalog number: 9065.4)
- Eukitt (O. Kindler, catalog number: 4023.1), store at room temperature
- Formaldehyde 37% (Roth, catalog number: P733.1)
- KCl
- KH2PO4
- NaCl
- NaH2PO4
- Nitric acid (Roth, catalog number: 2616.2)
- Paraformaldehyde (Serva, catalog number: 31628.02)
- Pyridine (AppliChem, catalog number: A0776.0500)
- Silver nitrate (MerckMillipore, catalog number: 1015120025)
- Sodium carbonate anhydrous (MerckMillipore, catalog number: 1063920500)
- Sodium hydroxide (MerckMillipore, catalog number: 1064981000)
- Sodium thiosulfate pentahydrate (J.T. Baker, catalog number: 3946-01)
- Sucrose (Sigma-Aldrich, catalog number: S9378-5KG)
- Tungstosilicic acid hydrate (MerckMillipore, catalog number: 1006590025)
- Xylene (Otto Fischar, catalog number: 27404)
- 10x PBS stock solution (see Recipes)
- 4% PFA for tissue (see Recipes)
- Incubating solution (see Recipes)
- Physical developing solution (see Recipes)
- Solution A
- Solution B
- Solution C
Equipment
- Filter unit (Corning, catalog number: 431153)
- Fume hood (Dueperthal, model: Typ 90)
- Glassware (rectangular staining dish with glass cover, removable glass slide rack that, dimensions: 91 mm x 71 mm x 60 mm, sold by e.g., VWR)
- Microscope (Zeiss, model: Axiophot)
- Microtome (ThermoFisher Scientific, model: HM 430)
- Paraffin embedding machine (Microm, model: HMP110)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Joseph, S., Werner, H. B. and Stegmüller, J. (2019). Gallyas Silver Impregnation of Myelinated Nerve Fibers. Bio-protocol 9(22): e3436. DOI: 10.21769/BioProtoc.3436.
- Joseph, S., Vingill, S., Jahn, O., Fledrich, R., Werner, H. B., Katona, I., Mobius, W., Mitkovski, M., Huang, Y., Weis, J., Sereda, M. W., Schulz, J. B., Nave, K. A. and Stegmuller, J. (2019). Myelinating glia-specific deletion of Fbxo7 in mice triggers axonal degeneration in the central nervous System together with peripheral neuropathy. J Neurosci 39(28): 5606-5626.
分类
神经科学 > 发育 > 组织学染色
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link