- EN - English
- CN - 中文
Super-resolution Microscopy at Cryogenic Temperatures Using Solid Immersion Lenses
固体浸没透镜应用于低温条件下的超分辨率显微镜
发布: 2019年11月20日第9卷第22期 DOI: 10.21769/BioProtoc.3426 浏览次数: 6724
评审: Gal HaimovichAnonymous reviewer(s)
Abstract
Our mechanistic understanding of cell function depends on imaging biological processes in cells with molecular resolution. Super-resolution fluorescence microscopy plays a crucial role by reporting cellular ultrastructure with 20-30 nm resolution. However, this resolution is insufficient to image macro-molecular machinery at work. A path to improve resolution is to image under cryogenic conditions, which substantially increases the brightness of most fluorophores and preserves native ultrastructure much better than chemical fixatives. Cryogenic conditions are, however, underutilized because of the lack of compatible high numerical aperture (NA) objectives. Here we describe a protocol for the use of super-hemispherical solid immersion lenses (superSILs) to achieve super-resolution imaging at cryogenic temperatures with an effective NA of 2.17 and resolution of ~10 nm.
Keywords: Super-resolution microscopy (超高分辨显微镜)Background
Fluorescence microscopy has for many years been one of the most important tools for understanding how biological systems are organized and how they function. Over the last decade, fluorescence microscopy has been revolutionized by the development of “super-resolution” methods that extend the limit of optical microscopy beyond the diffraction limit, providing unprecedented levels of information on the organization of molecular networks in cells. These methods include Structured Illumination Microscopy (SIM) (Gustafsson, 2000), Stimulated Emission Depletion Microscopy (STED) (Hell and Wichmann, 1994), and techniques based on the localization of individual fluorescent molecules with much better precision than the diffraction limit. The latter includes Photo-activated Localization Microscopy (PALM) (Betzig et al., 2006) and Stochastic Optical Reconstruction Microscopy (STORM) (Rust et al., 2006), referred to collectively in this protocol as Single Molecule Localization Microscopy (SMLM) techniques. Because of a requirement to stop motion within the cell when aiming for high resolution, super-resolution methods usually involve the use of chemical fixation, which has the potential to introduce artefacts that can be visible at the resolutions achieved (Whelan and Bell, 2015). This problem has long been recognized in the field of electron microscopy, where a different method is now routinely used to fix cells and preserve their structure: vitrification at cryogenic temperatures. In this method, samples are frozen using a cryogen, cooling rapidly to temperatures of -150 °C or below, resulting in the formation of amorphous ice, with the high rate of cooling preventing the formation of ice crystals (Dubochet and McDowall, 1981). SMLM techniques in particular should benefit from the use of cryogenic conditions. This is because the localization precision of fluorescent molecules is dependent on the number of photons emitted by the fluorophores (Thompson et al., 2002), and at cryogenic temperatures significantly more photons can be collected. Conventional widefield fluorescence microscopy at cryogenic temperatures is already used in Correlative Light and Electron Microscopy (CLEM) (de Boer et al., 2015) workflows, but the mismatch in resolution between optical microscopy and EM limits the value of the fluorescence data, which is usually restricted to locating general areas of interest. Hence, enabling cryogenic super-resolution fluorescence microscopy would be a significant advance for cryogenic CLEM workflows.
Given the advantages of vitrification over chemical fixation, it would be advantageous to apply it to super-resolution optical microscopy methods. However, its use has been severely limited for one major reason: super-resolution methods require the use of high numerical aperture (NA) objective lenses (NA > 1), and achieving this NA typically requires the use of oil immersion objectives, incompatible with cryogenic microscopy. A number of approaches have been taken to avoid this problem, including using non-immersion objectives with extremely bright fluorophores (Liu et al., 2015) and using special cryogenic immersion fluids and custom sample stages (Nahmani et al., 2017). However, these approaches have not been generally applied to the imaging of biological samples.
We have developed a low-cost, generally applicable method for super-resolution cryo-fluorescence microscopy that uses commercially available optical components and cryo-stage (Wang et al., 2019). For this, we use super-hemispherical solid-immersion lenses (superSILs). These lenses are made of high refractive index materials, and take the form of truncated spheres. In our application, their role is to take the place of immersion oil, and fill the gap between the sample and a conventional non-immersion objective. The combination of superSIL and air objective gives a high numerical aperture, dependent on the refractive index of the lens material (Terris et al., 1994; Zhang et al., 2007; Chen et al., 2013).
We have characterized the performance of superSILs for cryo-SMLM, showing that we are able to achieve a point spread function (PSF) size of around 150 nm, compared with around 330 nm for a cryogenic microscope with a conventional low NA air objective. This is because the combination of superSIL and objective achieves a NA of 2.17, much higher than the maximum NA of ~1.4 of oil-immersion objectives. The superSIL microscope achieves single molecule localization precisions of < 10 nm, and a lateral resolution of 12 nm (Wang et al., 2019). A major advantage of the superSIL method is that it can be achieved using readily available off-the-shelf components, with the possibility of easily adding the cryostage to microscopes commonly found in cell biology research laboratories. Moreover, superSIL assemblies can be cleaned and reused multiple times, making them an inexpensive resource. In this protocol, we focus on use of the superSIL system for super-resolution microscopy of bacteria, but also indicate how it might be applied to imaging of mammalian cells. A similar protocol could also be used to image other samples, such as purified protein complexes or vesicles. Used as described, the protocol provides a straightforward method for super-resolution imaging of biological samples at cryogenic temperatures, with the advantage of avoiding potential fixation artefacts. The resolution and localization precision achieved are significantly better than those routinely obtained in conventional room temperature SMLM microscopes. These advantages alone should make superSIL SMLM microscopy a valuable addition to the toolbox of cell biology researchers.
It is also possible to consider other potential advantages of the superSIL microscopy protocol. Firstly, because of the ultra-high NA, the resolution that can be achieved even at room temperature is significantly better than that obtained in conventional SMLM microscopes. The superSIL protocol therefore provides a simple low-cost method by which a basic fluorescence microscope could be converted to a high-performance super-resolution system. Our protocol describes use of the superSIL in an upright microscope, but the method could also be used in an inverted microscope by using a modified version of the superSIL assembly.
We believe that superSIL microscopy could have an important role to play in CLEM, helping to bridge the resolution gap between cryo-fluorescence microscopy and EM. We do not describe any CLEM approaches in this protocol, but possible methodologies could involve the transferring and imaging of thin lamellae produced by focused ion beam (FIB) milling under both superSIL and transmission EM modes, or the correlation of superSIL SMLM images with serial block-face scanning EM. Given the small size of the lens assembly, it may also be possible to incorporate a superSIL into the stage of an electron microscope, potentially permitting simultaneous or near-simultaneous imaging of the same sample with both light and electrons.
Materials and Reagents
- superSIL assembly
- Hyper Hemispheric Ball lens, Cubic Zirconia, 0.89 mm diameter x 0.73 mm thickness, λ\4 flatness (Knight Optical, LBB2018-C)
- Platinum Foil (Goodfellow, catalog number: PT000264 876-861-73)
- Cryo-compatible adhesive (Loctite Stycast, 2850 FT)
- Preparation of bacterial and fiducial solutions
- Flat cap centrifuge tubes, 50 ml (Fisherbrand, catalog number: 05-539-13)
- Eppendorf tubes, 2 ml (Eppendorf, catalog number: 0030 123.344)
- Tetraspeck 0.1 μm blue/green/orange/dark red beads (Molecular probes, Thermo Fisher Scientific, catalog number: T7279)
- Agar plate(s) with colonies of E.Coli expressing plasmid of interest
- LB medium granular (e.g., Melford, L24400-5000.0)
- 1 M HCl solution in water for pH adjustment (made up from HCl 37% ACS grade, 320331, Sigma-Aldrich)
- Selection antibiotics stock solutions in water (e.g., Kanamycin 25 mg/ml from powder-Sigma-Aldrich, K1377; Carbenicillin 100 mg/ml ready-made solution, Sigma-Aldrich, C1613)
- IPTG 1 M stock solution in water (made up from powder e.g., Fluorochem, M02726)
- PBS 1x pH 7.4 (Gibco, catalog number: 10010-015)
- Ultrapure deionized water (MilliQ or equivalent)
- LB medium (see Recipes)
- Cleaning of superSIL assemblies
- Clean glass slides (e.g., Fischer Scientific 16 x 38 mm microscope slides 244879)
- Filter paper (e.g., qualitative filter paper grade A, SLS 2036)
- Clean glass scintillation vials (e.g., Thermo Chromacol 20 ml vials 20-EPSVCA)
- superSIL assemblies (produced as described in Procedure A), cubic zirconia crystal NA 2.17
- Sulphuric acid ≥ 95% pure (e.g., Fischer Scientific S/9231/PB15)
- 30% Hydrogen Peroxide with inhibitor, ACS grade (e.g., Sigma-Aldrich, catalog number: 216763)
- At least 100 ml of MilliQ (or equivalent) water
- Piranha Solution (see Recipes)
- Plunge-freezing
- Ethane gas bottle
- Cleaned and glow discharged superSILs (prepared as described in Procedure D)
- Bacterial suspension (prepared as described in Procedure B)
- Fiducial suspension (prepared as described in Procedure C)
- Liquid nitrogen (at least a full 4 L dewar)
- 70% ethanol
- MilliQ water
Equipment
- superSIL assembly and microscope
- Ceramic hot plate (VWR, catalog number: 444-0624)
- Coordinate measuring machine (OGP, SmartScope ZIP 250)
- Cerna Microscope Body with Epi-Illumination Arm (Thorlabs, catalog number: CEA1350)
- Nikon D-FL Epi-Fluorescence Illuminator (Thorlabs, catalog number: CSE1000)
- Microscope cube assembly for Nikon TE2000 (Thorlabs, catalog number: TLV-TE2000)
- Single objective arm (Thorlabs, catalog number: CSA1100)
- Objective focusing module (Thorlabs, catalog number: ZFM2020)
- 3-axis controller and knob box for 1" Cerna stages (Thorlabs, catalog number: MCM3001)
- TIRF module comprising of X/Y translator and right-angle mirror mount (Thorlabs, ST1XY-D and KCB1/M)
- Translation stage (Prior, H117P2IX/G)
- Translation stage controller (Prior, ProScan III H31XYZEF)
- Image splitting module (Cairn, Twincam)
The following components are used in the microscope schematic illustrated in Figure 1. In principle, most popular commercial microscope stands could be modified to create a similar configuration since various adapter plates for the cryo-stage are available from Linkam Scientific Ltd and the Cairn Twincam can be purchased with c-mount threads on the input and output ports to suit most types of scientific camera.
Note: Check the emission spectra of your fluorophore or dye of choice at cryogenic temperature and select dichroic and emission filters to suit. The chromatic aberration between channels can be compensated for by changing the strength of lens l6 and axial translation of sCMOS camera 2.- l1: f = 30 mm achromatic doublet lens (Thorlabs, catalog number: AC254-030-A-ML)
- l2: f = 150 mm achromatic doublet lens (Thorlabs, catalog number: AC254-150-A-ML)
- l3: f = 100 mm achromatic doublet lens (Thorlabs, catalog number: AC254-100-A-ML)
- l4: f = 150 mm achromatic doublet lens (Thorlabs, catalog number: AC254-150-A-ML)
- l5: f = 200 mm 1x camera tube lens (Thorlabs, catalog number: WFA4100)
- l6: f = 500 mm B-BK7 plano-convex lens (Thorlabs, catalog number: LA1908)
- l7: 50x, 0.75 NA Microscope objective lens (Mitutoyo, M Plan Apo HR 378-814-4)
- p1: 30 μm pinhole (Thorlabs, P30D)
- t1: Top-hat beam shaping optic (TOPAG Lasertechnik GmbH, GTH-5-250-4-VIS)
- d1: Dichroic beam splitter (Semrock, Di-R405/488/561/635)
- d2: Dichroic beam splitter (Chroma, t565lpxr)
- f1: Quad-band emission filter (Semrock, FF01-446/523/600/677-25)
- f2: Green channel band-pass filter (Semrock, catalog number: FF03-525/50-25)
- f3: Red channel band-pass filter (Semrock, catalog number: FF01-593/40-25)
- q1: Achromatic quarter wave-plate (Thorlabs, AHWP10M-600)
- m1: Broadband dielectric mirror (Thorlabs, BB1-E02)
- Omicron LightHUB-6 containing four laser modules
- 405 nm 60 mW Phoxx diode laser
- 488 nm 200 mW Phoxx diode laser
- 561 nm 150 mW Cobolt Jive DPSS laser
- 642 nm 140 mW Phoxx diode laser
- Two back-illuminated sCMOS cameras with 18.7 mm chip sensor size (Teledyne Photometrics Ltd, 18.7 mm Prime 95B)
- Cryo-stage with autofill Dewar (Linkam, CMS196M and AUTOFILL)
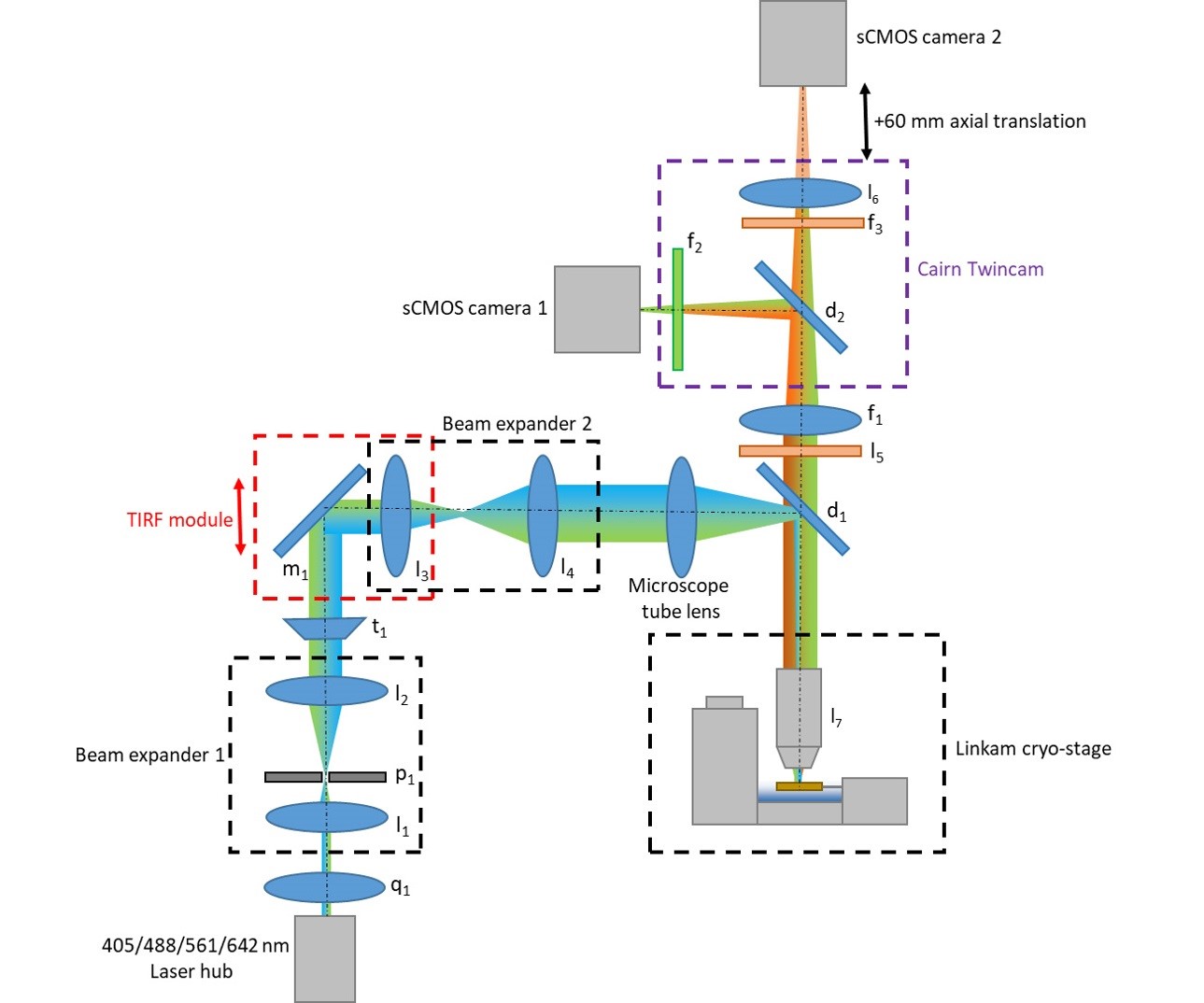
Figure 1. Microscope schematic detailing optics layout
- Preparation of bacterial and fiducial solutions
- Swinging bucket centrifuge (e.g., Beckman Coulter Allegra X-15R centrifuge)
- P1000, P100 and P10 pipettes and tips
- Autoclave (e.g., Prestige Medical Classic tabletop autoclave)
- Spectrophotometer (e.g., Amersham Biosciences Ultrospec 10 cell density meter)
- MSC cabinet (e.g., Scanlaf Mars Safety Class 2)
- Temperature-controlled, agitating incubator (e.g., New Brunswick Scientific Innova 44R)
- Vortex (e.g., IKA MS2 minishaker)
- Immersion probe pH meter (e.g., Hanna PH21)
- Cleaning of superSIL assemblies
- Chemical fume hood
- P1000 pipette and tips
- Glow discharger (e.g., Quorum GloQube)
- Inverted tweezers (e.g., Dumont N1)
- Timer
- Plunge-freezing
- FEI Vitrobot or equivalent plunge-freezer
- Flammable gas regulator (e.g., Air Liquide HBD-240-4-2.S)
- Gas leak detector (e.g., Everbuild Plumber’s P18 Gas Leak Detector)
- Inverted tweezers (e.g., Dumont N1)
- Curved tweezers (e.g., Dumont 7, 0130-7-PO)
- Blotting paper (e.g., Agar round filter paper for Vitrobot, 47000-100)
- P10 pipette and tips
- 1 L Thermos flask
- Funnel
- Tissue paper
- Grid boxes (e.g., SwissCI cryo-EM grid box MD16-104, Molecular Dimensions Limited)
- A 50 ml centrifuge tube attached to a piece of synthetic, non-absorbent twine
- Transport cryodewar (e.g., Statebourne Cryogenics OD-1)
- Handheld liquid nitrogen dewar (e.g., Worthington LD4)
- Storage cryodewar (e.g., Worthington VHC35)
- Hot plate or hair dryer
- Adequate personal protective equipment (PPE) for work with cryogenics (refer to local health and safety regulations at your facility)
- Optional: PFTE thread seal tape (e.g., Buffalo 12 m x 12 mm x 0.075 mm BS7786: 2006 Grade L)
- Optional: Local Exhaust Ventilation (LEV) inlet
- Optional: orientable tabletop lamp
- FEI Vitrobot or equivalent humidity controller
Software
- Micro-Manager v1.4.23 20161114 (Edelstein et al., 2014)
- Omicron Control Centre v2.1.4
- ImageJ version v1.52h (Schindelin et al., 2015)
- ThunderSTORM v1.3-2014-11-08 plugin (Ovesný et al., 2014)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Bateman, B. C., Zanetti-Domingues, L. C., Moores, A. N., Needham, S. R., Rolfe, D. J., Wang, L., Clarke, D. T. and Martin-Fernandez, M. L. (2019). Super-resolution Microscopy at Cryogenic Temperatures Using Solid Immersion Lenses. Bio-protocol 9(22): e3426. DOI: 10.21769/BioProtoc.3426.
分类
生物物理学 > 显微技术 > 低温显微镜技术
细胞生物学 > 细胞成像 > 超分辨率成像
细胞生物学 > 细胞成像 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link