- EN - English
- CN - 中文
Implementing VIPER for Imaging Cellular Proteins by Fluorescence Microscopy
VIPER用于荧光显微镜中细胞蛋白成像
发布: 2019年11月05日第9卷第21期 DOI: 10.21769/BioProtoc.3413 浏览次数: 5779
Abstract
Genetically-encoded tags are useful tools for multicolor and multi-scale cellular imaging. Versatile Interacting Peptide (VIP) tags, such as VIPER, are new genetically-encoded tags that can be used in various imaging applications. VIP tags consist of a coiled-coil heterodimer, with one peptide serving as the genetic tag and the other (“probe peptide”) delivering a reporter compatible with imaging. Heterodimer formation is rapid and specific, allowing proteins to be selectively labeled for live-cell and fixed-cell imaging. In this Bio-Protocol, we include a detailed guide for implementing the VIPER technology for imaging receptors on live cells and intracellular targets in fixed cells. This protocol is complemented by two other Bio-Protocols outlining the use of VIPER (Doh et al., 2019a and 2019b).
Keywords: Protein tag (蛋白标签)Background
Fluorescence microscopy (FM) enables researchers to observe the architecture and assembly of proteins in cells dynamically and in multicolor (Liu et al., 2015; Valm et al., 2017; Guo et al., 2018; Liu et al., 2018). Fluorescence imaging relies on strategies for labeling target proteins with bright, fluorescent reporter molecules. Labeling can be achieved in a number of ways (Giepmans et al., 2006; Crivat and Taraska, 2012; Vandemoortele et al., 2019), including immunolabeling, fluorescent proteins (e.g., green fluorescent protein) (Shaner et al., 2005; Snapp, 2009), chemical stains (e.g., DAPI, MitoTracker, or phalloidin conjugates) (Cottet-Rousselle et al., 2011; Bucevičius et al., 2018), and self-labeling tags (Keppler et al., 2003; Gautier et al., 2008; Los et al., 2008). The most useful tags can be used to deliver diverse chemical reporters with optimal properties, such as spectrally-distinct colors, high quantum yield and extinction coefficient (“brightness”), and photostability (Dempsey et al., 2001; Li and Vaughan, 2018). Tags that can bind to a variety of bright fluorophore ligands include the SNAP tag (Keppler et al., 2003; Gautier et al., 2008), Halo tag (Los et al., 2008), TMP tag (Miler et al., 2005), and FAPs (Szent-Gyorgyi et al., 2008). However, these protein tags are large (18-33 kDa), which can change protein folding, trafficking, and function (Brock et al., 1999; Costantini and Snapp, 2013; Huang et al., 2014; Johnson et al., 2015). A few peptide tags have been described for cell imaging, as exemplified by the tetracysteine tag, but these can have non-specific interactions and limited color choices (Griffin et al., 1998; Gaietta et al., 2002; Cohen et al., 2011; Liu et al., 2014).
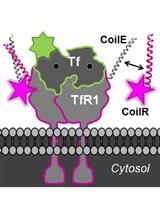
An alternative approach is to use a peptide tag that forms a heterodimeric coiled-coil with a reporter peptide. This is the approach that we (Zane et al., 2017; Doh et al., 2018) and others (Tsutsumi et al., 2009; Nomura et al., 2010; Tsutsumi et al., 2011; Reinhardt et al., 2014 and 2015; Lotze et al., 2018) have used to label cellular proteins (Lotze et al., 2016; Yano and Matsuzaki, 2019). One advantage of this approach is that the genetic tag is small–just 4 to 7 kDa. A second advantage is that reporter peptide labeling is typically restricted to the cell surface, which is useful for labeling and tracking transmembrane receptors (Yano et al., 2008) (i.e., in pulse-chase experiments (Doh et al., 2018; Lotze et al., 2018)). We named our coiled-coil tags Versatile Interacting Peptide (VIP) tags. Our first tag, VIP Y/Z, enabled the selective fluorescent labeling of target proteins in cell lysates and on live cells (Zane et al., 2017). Next we described VIPER, which is comprised of a CoilE tag and a CoilR probe peptide. We showed that the probe peptide can be customized to the imaging application by conjugation to one of a number of reactive fluorophores and small molecules (i.e., biotin). VIPER labeled sub-cellular structures in fixed cells and transmembrane receptors on live cells. Proteins could be imaged by FM or correlative light and EM (CLEM) (Doh et al., 2018).
For any genetic tag, it is important to insert the tag at a location in the amino acid sequence where it will not interfere with the binding interactions, localization, folding, or function of the protein of interest. It is beyond the scope of this paper to dictate the location of the genetic tag for all feasible protein targets. We recommend reading Erik Snapp’s paper “Design and Use of Fluorescent Fusion Proteins in Cell Biology” for a discussion on choosing a tag insertion site (Snapp, 2005). For VIP tags, we offer the following suggestions and recommendations for tag placement.
This protocol includes methods for implementing VIPER for imaging cellular proteins by FM. These imaging experiments are performed on transfected mammalian tissue culture cells. In Procedure A we provide a transfection protocol for introducing plasmid DNA encoded VIP-tagged constructs into cells. In Procedure B, we describe labeling a transmembrane receptor on the surface of living cells. In Procedure C, we describe methods for imaging intracellular targets in fixed cells. This publication is complemented by Doh et al. (2019a), which outlines how to generate CoilR probe peptide, and Doh et al. (2019b), which discusses using VIPER to image proteins by CLEM. A decision tree for implementing these methods is provided in Figure 1.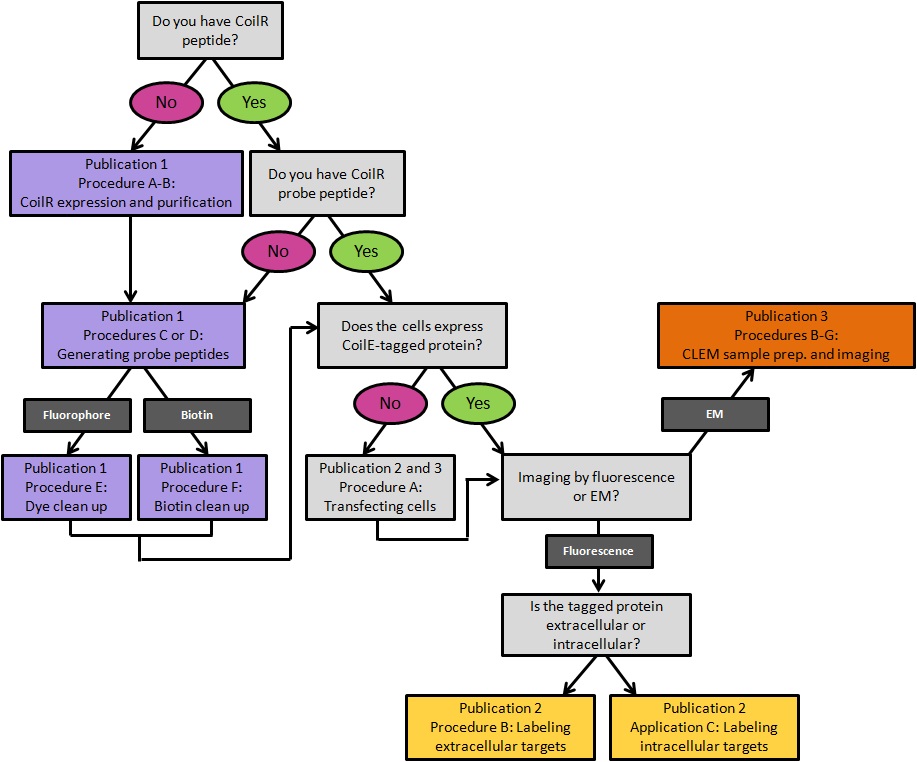
Figure 1. A decision tree for implementing VIPER for labeling cellular proteins. Procedures are color-coded by the publication in which they appear. Publication 1: Doh et al., 2019a; Publication 2: this article; Publication 3: Doh et al., 2019b.
Materials and Reagents
Note: “*” indicates a brand that is critical to the success of the experiment.
Materials
- Polystyrene 10 cm tissue culture dish (Thermo Scientific, NuncTM, catalog number: 12565020)
- Polystyrene 6-well tissue culture plate (Thermo Scientific, BioLiteTM, catalog number: 12-556-004)
- 8-well chambered coverglass with #1.5 glass (i.e., Cellvis, catalog number: C8-1.5H-N or Thermo Scientific, NuncTM Lab-TekTM, catalog number: 155411)
- LDPE 500 ml squeeze wash bottle (Thermo Scientific, catalog number: 24010500)
- Transfer bulb pipette (VWR, catalog number: 16001-182)
- U-2 OS cells (ATCC, catalog number: HTB-96)
- CHO TRVb cells (Courtesy of Prof. Timothy McGraw, Cornell University, Ithaca, New York) (McGraw et al., 1987)
- Plasmid DNA of VIP-tagged protein (Generated by the user)
- mEmerald-actin-C18 (Addgene, catalog number: 53978)
- Human transferrin Alexa FluorTM 488 conjugate; Tf-AF488 (Invitrogen, catalog number: T13342)
- *LipofectamineTM 2000 (Thermo Scientific, catalog number: 11668019)
- Ham’s F-12 Medium (Life Technologies, GibcoTM, catalog number: 11765062)
- McCoy’s 5A Medium (Life Technologies, GibcoTM, catalog number: 16600082)
- Dulbecco’s phosphate-buffered saline without calcium or magnesium; DPBS (GibcoTM, catalog number: 14190144)
- Trypsin-EDTA (0.25%) (Life Technologies, GibcoTM, catalog number: 25200056)
- Fetal bovine serum; FBS (GE, HycloneTM, catalog number: SH30910.03)
- Opti-MEM (Life Technologies, GibcoTM, catalog number: 31985070)
- Hoechst 33342 nucleic acid stain (Invitrogen, catalog number: H1399)
- Bovine serum albumin (BSA) fraction V (Research Products International, catalog number: A30075100.0)
- Immersion oil (Carl Zeiss ImmersolTM 518 F, catalog number: 4449620000000)
- Sodium azide (Sigma-Aldrich, catalog number: 71289-5G)
- Triton X-100 (Sigma-Aldrich, catalog number: 93443-100ML)
- Saponin (Sigma-Aldrich, catalog number: 558255100GM)
- Ham’s F12 media
- Glycerol (Thermo Fisher, Fisher BioReagentsTM, catalog number: BP229 1)
- Tris (Thermo Fisher, Fisher BioReagentsTM, catalog number: BP152-500)
- Sucrose (Thermo Fisher, Fisher Chemical, catalog number: D16-500)
- Live Cell Block Solution (see Recipes)
- Mounting Medium (see Recipes)
- Fixed Cell Block Solution (see Recipes)
Equipment
- Hemocytometer (Hausser Scientific, catalog number: 1475)
- Humidified CO2 Incubator (New Brunswick Galaxy 170S, catalog number: C0170S-120-0000)
- Tissue culture hood (Thermo Scientific, 1300 Series A2)
- Tissue culture inverted light microscope (Carl Zeiss, Zeiss Primovert)
- Spinning disk confocal fluorescence microscope (Zeiss Yokogawa CSU-X1 on Zeiss AxioObserver)
- Line scanning (Airyscanning) fluorescence microscope (Zeiss LSM880 on inverted microscope stand)
Software
- Fiji (ImageJ Version 2.0.0-rc-46) (Schindelin et al., 2012)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Doh, J. K., Enns, C. A. and Beatty, K. E. (2019). Implementing VIPER for Imaging Cellular Proteins by Fluorescence Microscopy. Bio-protocol 9(21): e3413. DOI: 10.21769/BioProtoc.3413.
分类
癌症生物学 > 增殖信号转导 > 细胞生物学试验 > 化学抗性
细胞生物学 > 细胞成像 > 荧光
生物化学 > 蛋白质 > 标记
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link