- EN - English
- CN - 中文
Generation of CoilR Probe Peptides for VIPER-labeling of Cellular Proteins
用于细胞蛋白VIPER标记的CoilR肽探针的制备
发布: 2019年11月05日第9卷第21期 DOI: 10.21769/BioProtoc.3412 浏览次数: 5523
评审: Samantha E. R. DundonMartin V KolevAnonymous reviewer(s)
Abstract
Versatile Interacting Peptide (VIP) tags are a new class of genetically-encoded tag designed for imaging cellular proteins by fluorescence and electron microscopy. In 2018, we reported the VIPER tag (Doh et al., 2018), which contains two elements: a genetically-encoded peptide tag (i.e., CoilE) and a probe peptide (i.e., CoilR). These two peptides deliver contrast to a protein of interest by forming a specific, high-affinity heterodimer. The probe peptide was designed with a single cysteine residue for site-specific modification via thiol-maleimide chemistry. This feature can be used to attach a variety of biophysical reporters to the peptide, including bright fluorophores for fluorescence microscopy or electron-dense nanoparticles for electron microscopy. In this Bio-Protocol, we describe our methods for expressing and purifying recombinant CoilR. Additionally, we describe protocols for making fluorescent or biotinylated probe peptides for labeling CoilE-tagged cellular proteins. This protocol is complemented by two other Bio-Protocols outlining the use of VIPER (Doh et al., 2019a and 2019b).
Keywords: Peptide (肽)Background
Fluorescence microscopy (FM), electron microscopy (EM), and correlative light and EM (CLEM) enable investigations into the multi-protein complexes and macromolecular interactions that mediate normal and disease-associated cellular functions. However, multiscale microscopy is restricted by the shortage of methods for attaching FM-, EM-, and CLEM-compatible reporter chemistries to target proteins. Additionally, there are few methods for protein labeling that facilitate switching between imaging systems. As a result, most multiscale imaging studies obtain protein-specific contrast with immunolabeling. However, there are known drawbacks to immunolabeling. The large size of antibodies reduces localization precision, and labeling protocols can disrupt cellular ultra-structure (Schnell et al., 2012). Scarce proteins and rare interactions can elude detection unless immunolabeling is efficient (Griffiths and Hoppeler, 1986; Schnell et al., 2012; Griffiths and Lucocq, 2014). Many antibodies have poor target specificity and cross-reactivity (Berglund et al., 2008; Bordeaux et al., 2010; Baker, 2015; Bradbury and Pluckthun, 2015), which can result in misleading observations.
The central obstacle that has limited progress in multiscale microscopy is the shortage of genetic tags for labeling proteins. Most tags were developed for FM (Liu et al., 2015), with the most commonly used tags being fluorescent proteins [e.g., GFP] (Tsien, 1998; Cranfill et al., 2016; Rodriguez et al., 2017). By comparison, there are few genetic tags for EM or CLEM (Ellisman et al., 2012). We saw this as an opportunity to create a new class of genetically-encoded peptide tags for multiscale microscopy (Zane et al., 2017; Doh et al., 2018). We named this technology Versatile Interacting Peptide (VIP) tags (Figure 1). VIP tags consist of a heterodimeric coiled-coil between a genetically-encoded peptide tag and a reporter-conjugated peptide (“probe peptide”). Binding is driven by a hydrophobic interface and inter-strand salt bridges between the two coils. Initially we reported VIP Y/Z, which was used to label cellular proteins with fluorophores and Qdots (Zane et al., 2017). This pair consists of a heterodimeric CoilY-CoilZ pair with a reported dissociation constant (KD) of less than 15 nM (Reinke et al., 2010). Either CoilY or CoilZ could serve as the genetically-encoded tag. In 2018, we reported the VIPER tag, which enables high-affinity labeling of proteins for imaging by FM and CLEM (Doh et al., 2018). Binding between the CoilE tag and the CoilR probe peptide to form VIPER is specific and nearly irreversible [KD ~10-11 M] (Moll et al., 2001).
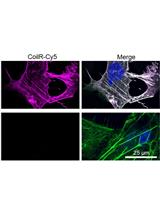
Figure 1. Versatile interacting peptide (VIP) tags are a new technology for imaging proteins by FM, EM or CLEM. VIPER labeling of transferrin receptor 1 (TfR1) is mediated by heterodimer formation between the CoilE tag and a fluorescent CoilR probe peptide. Fluorescent micrograph: VIPER-tagged TfR1 labeled with CoilR-Cy5 (magenta) and colocalized with fluorescent transferrin (Tf-AF488; green) at the cell surface of transfected CHO TRVb cells (63x magnification). Magenta-green signal overlap appears white and nuclei are blue.
Figure 2. VIP tags are a versatile technology for multi-scale microscopy. After a target protein is tagged, it can be labeled using a variety of probe peptides selected for the particular application.
For VIP tags, the versatility is imparted by the customizable probe peptide. After introduction of the CoilE tag onto a target protein, the protein can be labeled with one of many different reporters attached to CoilR (Figure 2). For example, we imaged the transmembrane receptor, TfR1-CoilE, with CoilR-BODIPY, CoilR-Cy5 (see Figure 1), and CoilR-biotin (Doh et al., 2018). In other words, the probe peptide can be customized for different studies or imaging systems without changing the genetic tag. This is possible because CoilR encodes a single cysteine residue for site-specific modification via thiol-maleimide chemistry. The CoilR probe peptide can be bioconjugated to a variety of probes, including fluorophores, small molecules (e.g., biotin), or nanoparticles. Many companies sell thiol-reactive probes, which makes this conjugation reaction accessible to labs without synthetic chemistry expertise. For more information on bioconjugation reactions, we recommend reading Hermanson’s Bioconjugate Techniques (Hermanson, 2013).
In this Bio-Protocol, we provide methods for making CoilR probe peptides that can be used for VIPER-labeling of cellular proteins for imaging by FM or EM. The CoilR peptide and the CoilE tag sequences are provided in Table 1. As described in prior work (Doh et al., 2018), we used gene assembly PCR to enable the recombinant expression of probe peptides in E. coli. The method for peptide expression is described in Procedure A. CoilR was designed to interact with CoilE via an optimized alpha-helical coil-coil, as originally described by Vinson and coworkers (Moll et al., 2001). We included a hexahistidine tag at the C-terminus of CoilR for purification by immobilized metal affinity chromatography (IMAC) (Hochuli et al., 1987); this is described in Procedure B.
Table 1. Sequences of CoilR and CoilE
§Italics: Linker sequence; Bold: Peptide coil; C: Cysteine (conjugation site).
‡Heptad position: Residues a and d form a hydrophobic interface, residues at e and g form inter-strand salt bridges.
Procedures C and D describe thiol-maleimide reactions to label CoilR with a small molecule reporter. In Procedure C, we describe the method that we used to generate probe peptides in our prior work (Doh et al., 2018). In Procedure D, we adapted a method described by Weiss and coworkers for solid state-based labeling of peptides (Kim et al., 2008). Lastly, we include methods for purifying fluorophore-labeled (Procedure E) or biotinylated (Procedure F) probe peptide. This Bio-Protocol is accompanied by two companion articles, which include detailed methods for imaging VIPER-labeled cellular proteins by FM (Doh et al., 2019a) and CLEM (Doh et al., 2019b) (Figure 3). 
Figure 3. A decision tree for implementing VIPER. Procedures are color-coded by the publication in which they appear. Methods in this publication are color-coded purple. Methods in Doh et al., 2019a are yellow and methods in Doh et al., 2019b are orange. Publication 1: this article; Publication 2: Doh et al., 2019a; Publication 3: Doh et al., 2019b).
Materials and Reagents
Note: “*” indicates a brand that is critical to the success of the experiment.
Materials
- Universal pipette tips (USA Scientific TipOneTM, catalog numbers: 1112-1770, 1163-1730, and 1121-3812)
- Microcentrifuge tubes, 1.5 ml (Thermo Scientific, catalog number: 02-682-002)
- Sterile serological pipettes (Thermo Scientific, catalog number: 13-678-11D + E)
- Sterile 14 ml culture tubes (Corning, FalconTM, catalog number: 352059)
- Disposable polystyrene spectrophotometer cuvettes (Thermo Scientific, catalog number: 14-955-127)
- Conical 50 ml tubes (Thermo Scientific, NuncTM, catalog number: 12-565-270)
- Chromatography column (Bio-Rad, Econo-PacTM, catalog number: 7321010)
- Ring stand (Fisher, catalog number: 11-474-207)
- Adjustable ring stand clamps (United Scientific Supplies, catalog number: CLHD03)
- Molecular weight cut off (MWCO) 3 kDa filters (Sigma-Aldrich, Amicon UltraTM, catalog number: UFC900324)
- Quartz 10.00 mm cuvette (Hellma Analytics, Ultra-Micro Cell, catalog number: 105-250-15-40)
- Pipettes (e.g., Rainin Pipet-LiteTM XLS, catalog numbers: 17014407, 17014411, 17014412, and 17014413)
- Glass 2 L Erlenmeyer flask (Corning, PyrexTM, catalog number: 49802L)
Reagents
- Anti-biotin HRP antibody (Jackson Immunoresearch, catalog number: 200-032-211)
- Streptavidin-HRP (Thermo Scientific, catalog number: ENN100)
- pET28b(+)_CoilR [Available by MTA from OHSU or made as published (Doh et al., 2018)]
- BL21 (DE3) E. coli (New England Biolabs, catalog number: C2527I)
- Glycine (Thermo Scientific, Fisher BioReagentsTM, catalog number: BP381-500)
- SOC outgrowth media (New England Biolabs, catalog number: C2527I)
- Miller Luria-Bertani (LB) agar (BD DifcoTM, catalog number: 244520)
- Miller LB broth (BD DifcoTM, catalog number: BD 244610)
- 2X YT (Thermo Scientific, Fisher BioReagentsTM, catalog number: BP9743500)
- Kanamycin sulfate (Thermo Scientific, Fisher Chemical, catalog number: BP906-5)
- IPTG (GoldBio, catalog number: I2481C5)
- *Ni-NTA agarose (Qiagen, catalog number: 30230)
- *Pierce Monomeric Avidin Agarose (Thermo Scientific PierceTM, catalog number: 20228)
- Sodium Phosphate Monobasic Anhydrous (Thermo Scientific, Fisher BioReagentsTM, catalog number: BP329-500)
- Urea (Thermo Scientific, Fisher BioReagentsTM, catalog number: U15 3)
- Tris Base (Thermo Scientific, Fisher BioReagentsTM, catalog number: BP152 5)
- Tris HCl (Thermo Scientific, Fisher BioReagentsTM, catalog number: BP153 1)
- NaCl (Thermo Scientific, Fisher BioReagentsTM, catalog number: BP358-1)
- Glycerol (Thermo Scientific, Fisher BioReagentsTM, catalog number: BP229-1)
- Imidazole (ACROS Organics, catalog number: AC39674-1000)
- Coomassie Brilliant Blue R-250 (Thermo Scientific, catalog number: 20278)
- Methanol (Thermo Scientific, Fisher Chemical, catalog number: A412)
- Acetone (Thermo Scientific, Fisher Chemical, catalog number: A18)
- Nitrogen gas
- Ammonium sulfate (EMD Millipore, catalog number: AX1385-1)
- TCEP-HCl (GoldBio, catalog number: TCEP10)
- Dithiothreitol (DTT) (Thermo Scientific, Molecular ProbesTM, catalog number: D1532)
- TC-grade DMSO (Sigma-Aldrich, catalog number: D2650-5X10ML)
- *Sulfo-Cy5-maleimide (Lumiprobe, catalog number: 23380)
- *Biotin-PEG2-maleimide (Thermo Scientific, catalog number: 21901BID)
- D-Biotin (Ark Pharma, catalog number: AK-44010)
- Pierce BCA assay kit (Thermo Fisher Scientific, catalog number: 23227)
- 12% Bis-Tris polyacrylamide protein gels (Bio-Rad CriterionTM XT, catalog number: 3450119)
- MES (Thermo Scientific, Fisher BioReagentsTM, catalog number: BP300-100)
- NaH2PO4 (Sigma-Aldrich, catalog number: S3139-250G)
- Ponceau Red (Thermo Scientific, Fisher BioReagentsTM, catalog number: BP103-10)
- NaOH (Thermo Scientific, Fisher BioReagentsTM, catalog number: BP359-500)
- Buffer B (Ni-NTA peptide purification) (see Recipes)
- Buffer C (Ni-NTA peptide purification) (see Recipes)
- Buffer E (Ni-NTA peptide purification) (see Recipes)
- MES running buffer (see Recipes)
- TCEP/SDS Loading Dye (5x) (see Recipes)
- Coomassie Stain (see Recipes)
- Destain Solution (see Recipes)
- Tris-Buffered Saline (TBS) (see Recipes)
- TBS Urea (see Recipes)
- 0.5 M TCEP (see Recipes)
- TBS Urea Binding Buffer (see Recipes)
- Solid state-based labeling (SSL) Buffer, pH 7.5 (see Recipes)
- 1 M DTT (see Recipes)
- TBS Urea Imidazole (see Recipes)
- Biotin Buffer (see Recipes)
- Regeneration buffer (see Recipes)
Equipment
- Electronic pipettor (Eppendorf EasypetTM, catalog number: 4430000018)
- -20 °C freezer (Thermo Scientific, RevcoTM, catalog number: 13 990 206)
- Incubator and shaker (New Brunswick ExcellaTM E24, catalog number: M1352-0010)
- Spectrophotometer (Eppendorf, Biophotometer Plus, catalog number: 6132)
- Rotisserie (Thermo Scientific, catalog number: 400110Q)
- Sonifier (Branson UltrasonicsTM, catalog number: 101063198R)
- Sonifier 1/8 inch micro-tip (Branson UltrasonicsTM, catalog number: 22-309796)
- Refrigerated centrifuge (Thermo Scientific, Sorvall Legend XTR Centrifuge, catalog number: 75211731)
- Microcentrifuge (Eppendorf, catalog number: 022620304)
- Heat block (Fisher, IsotempTM, catalog number: 88-860-022)
- Electrophoresis cell (Bio-Rad CriterionTM, catalog number: 165-6001)
- Power supply (Bio-Rad PowerPacTM HC, catalog number: 1645052)
- Plate reader (Tecan Infinite M200 Pro, catalog number: 30050303)
- (Optional) Fluorescence and western blot imager (i.e., GE Healthcare AmershamTM Typhoon 5 multimode scanner, catalog number: 29187191 or Protein Simple, FluorChem Q)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Doh, J. K., Tobin, S. J. and Beatty, K. E. (2019). Generation of CoilR Probe Peptides for VIPER-labeling of Cellular Proteins. Bio-protocol 9(21): e3412. DOI: 10.21769/BioProtoc.3412.
分类
癌症生物学 > 增殖信号转导 > 细胞生物学试验 > 化学抗性
细胞生物学 > 细胞成像 > 荧光
生物化学 > 蛋白质 > 标记
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link