- EN - English
- CN - 中文
Intracerebral Injection of Streptozotocin to Model Alzheimer Disease in Rats
一种用于链脲佐菌素诱导的大鼠阿尔兹海默症建模的立体定向手术
(*contributed equally to this work) 发布: 2019年10月20日第9卷第20期 DOI: 10.21769/BioProtoc.3397 浏览次数: 8210
评审: Edgar Soria-GomezShaarika SarasijaSébastien Gillotin

相关实验方案
基于 rAAV-α-Syn 与 α-Syn 预成纤维共同构建的帕金森病一体化小鼠模型
Santhosh Kumar Subramanya [...] Poonam Thakur
2025年12月05日 1633 阅读
Abstract
Animal models have promoted meaningful contribution to science including Alzheimer’s disease (AD) research. Several animal models for AD have been used, most of them related to genetic mutations observed in familial AD. However, sporadic form of AD, also named late-onset is the most frequent form of the disease, which is multifactorial, being influenced by genetic, environmental and lifestyle factors. Here, we describe a protocol of an AD-like pathology of the sporadic form using Wistar rats by a single bilateral intracerebroventricular (icv) injection of streptozotocin (STZ, 2 mg/kg). Icv injection of STZ induces brain resistance to insulin and other pathological alterations related to those observed in AD, such as cognitive impairment and accumulation of phosphorylated tau protein and β-amyloid in the brain. Thus, icv injection of STZ is a useful tool to investigate the pathological mechanisms and the metabolic alterations involved in AD and to propose new therapeutic approaches and neuroprotective drugs.
Keywords: β-amyloid (β-淀粉样蛋白)Background
Alzheimer’s disease (AD) is the most common cause of senile dementia. One to five percent of AD cases are familial and all the other cases are late-onset also known as sporadic AD. The understanding of sporadic AD has progressed substantially from studies using an AD animal model created by intracerebroventricular (icv) injection of streptozotocin (STZ) (Mayer et al., 1990; Lannert and Hoyer, 1998).
STZ is a glucosamine-nitrosourea derived from soil microbe Streptomyces achromogenes. The icv injection of STZ impairs cerebral glucose and energy metabolism, which may be a central event for triggering neurodegeneration in AD (Nitsch and Hoyer, 1991; Plaschke and Hoyer, 1993; Duelli et al., 1994). The mechanisms of icv STZ-induced AD-like pathology were recently reviewed by Grieb (2016) and Kamat et al. (2016). The pathological alterations induced by icv injection of STZ are analogous to the features of sporadic AD, which include increase in β-amyloid protein and hyperphosphorylation of tau protein in several brain structures, intensive neuronal death, increase of the brain ventricles, cerebral inflammation, cognitive deficit, anxiety- and depression-like behaviors, decrease in social interaction, increase of core body temperature and cold-avoidance behavior (Kraska et al., 2012; Santos et al., 2012; Chen et al., 2013; Mehla et al., 2013; Knezovic et al., 2015; Dehghan-Shasaltaneh et al., 2016; Crunfli et al., 2018; Moreira-Silva et al., 2018; Motzko-Soares et al., 2018; Vicente et al., 2018; Amani et al., 2019; Crunfli et al., 2019). STZ is usually diluted in saline, citrate buffer, or artificial cerebrospinal fluid, and administrated in a single or multiple injections in a range of 1-3 mg/kg, unilaterally or bilaterally in the lateral ventricles of the brain (Grunblatt et al., 2007; Kraska et al., 2012; Mehla et al., 2013; Dehghan-Shasaltaneh et al., 2016; Adeli et al., 2017). The pathological alteration induced by STZ are progressive and time- and dose-dependent. Thus, icv-STZ injection is characterized by an acute-fast impairment in the memory, followed by partial recovery, and then, a chronic-slow memory impairment (Knezovic et al., 2015). Also, higher doses of STZ promote more severe and fast neurodegeneration and more evident cognitive deficit (Kraska et al., 2012; Knezovic et al., 2015, Dehghan-Shasaltaneh et al., 2016).
Here, we describe a step-by-step protocol used in our laboratory, as summarized in Figure 1. Approximately 30 days from the icv-STZ injection at a dose of 2 mg/kg, clear cognitive impairment and neurodegeneration were observed (Moreira-Silva et al., 2018; Motzko-Soares et al., 2018; Vicente et al., 2018). The protocol used in our laboratory was standardized based on evidences that show: (1) STZ at high doses (≥ 3 mg/kg) promotes alteration on locomotor activity (Dehghan-Shasaltaneh et al., 2016), and also induce acute neurotoxic effect with fast severe neurodegeneration that differs from the slow neurodegenerative progress observed in sporadic AD (Kraska et al., 2012); (2) STZ at lower doses (≤ 1 mg/kg) promotes a very slow neurodegenerative progress and is more appropriate to study neuroprotective drugs (Kraska et al., 2012); (3) an intermediate dose of STZ (around 2 mg/kg) was previously reported as the most efficient dose to modeling a sporadic AD-like pathology (Dehghan-Shasaltaneh et al., 2016); (4) multiple injections of STZ increases animal mortality (Mehla et al., 2013); and (5) STZ shows better stability in solution at pH 4.5 than at neutral pH (Junod et al., 1967). 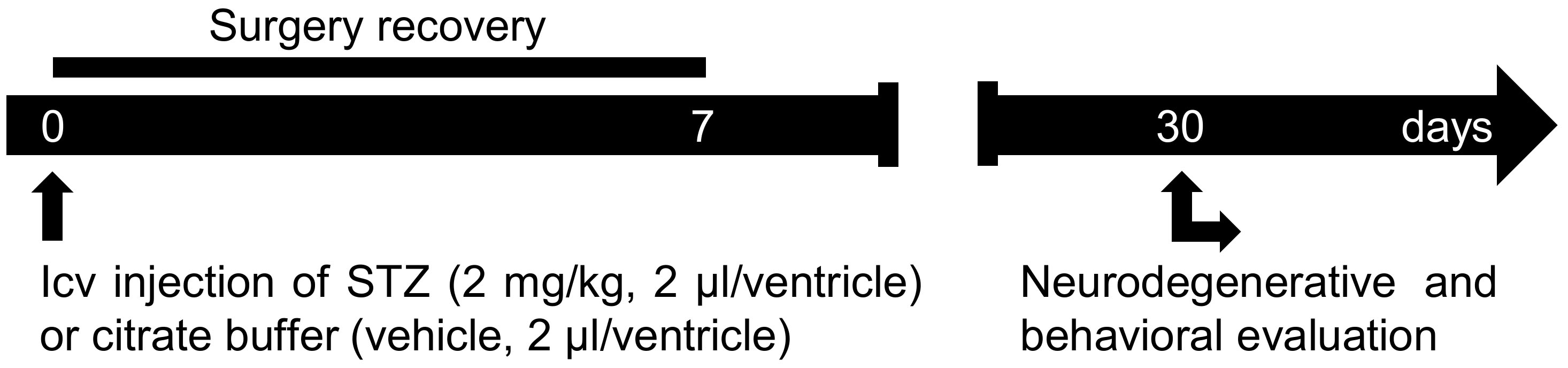
Figure 1. Protocol scheme. STZ (2 mg/kg, 2 µl/ventricle) or vehicle (2 µl/ventricle) is injected in both lateral ventricles of the rat’s brain. In the first week post-surgery, animal recovery should be evaluated. After 30 days of icv-STZ injection, animal behavior and the neurodegenerative alterations induced by icv-STZ can be evaluated.
Materials and Reagents
- Animals
Male Wistar rats (3 to 4 months old, 350-400 g)
Notes:- Institutional animal ethics protocol approval is required to perform the procedure described in this protocol. Personal protective equipment is recommended.
- If the rats are obtained from another source, allow them to habituate to the new colony for at least 3 days before undergoing surgery.
- In our lab, it was observed that rats under 300 g of body mass were more susceptible to body mass loss after an icv-STZ injection.
- Here we recommend male rats since female rats present less sensitivity to STZ-induced cognitive impairment compared to male rats (Bao et al., 2017). Moreover, female rats display differences in some AD typical biomarkers compared to those observed in human. However, these differences were observed in Sprague-Dawley rats. More studies testing the sex differences related to AD susceptibility and progression in other rodent species and strains are needed to better understand how the hormonal modulation could affect the disease progression. As AD is more prevalent between women than men, the fact that STZ models are generally applied only to male rodents is a bias that restricts the representativeness of the model (Bao et al., 2017).
- STZ and vehicle preparation
- Spatula (Sigma-Aldrich, catalog number: Z648299)
- Beaker 50 ml (Qualividros, catalog number: 59930)
- Pipette tips 0.1-10 µl (VWR, catalog number: 53509-138)
- Microcentrifuge tube 0.5 ml (Eppendorf, catalog number: 0030124537)
- STZ (Santa Cruz Biotechnology, catalog number: sc-200719)
- Sodium citrate (Sigma-Aldrich, catalog number: W302600)
- Sodium chloride (Sigma-Aldrich, catalog number: S7653)
- Hydrochloric acid (Sigma-Aldrich, catalog number: 320331)
- Sodium hydroxide (Sigma-Aldrich, catalog number: S8045)
- Double distilled water
- 0.05 M Citrate buffer (see Recipes)
- Surgery
- Aluminum foil
- Scissors (Roboz, catalog number: RS-6700)
- Tissue forceps (Roboz, catalog number: RS-8166 or RS-5242)
- Suture Needle (Aspen Surgical, Richard-Allan® needle, catalog number: 200016)
- Suture thread (Braunamid, catalog number: F1185047)
- Cotton gauze (Fisherbrand, catalog number: 22-362178)
- Micropipette (Gilson Inc., catalog number: F81022)
- 1 ml syringe (BD, catalog number: 309628)
- 22 G needle (BD, catalog number: 305159)
- 25 G needle (BD, catalog number: 305122)
- 24 G intravenous catheter (Terumo, catalog number: SR-OX2419CA)
- Polyethylene tubing PE-10 (Scientific Commodities Inc., catalog number: BB31695-PE/1)
- Microsyringe 10 µl (Hamilton, catalog number: 80300)
- Ketamine (Syntec)
- Xylazine (Syntec)
- (Optional ) Lidocaine and epinephrine
- Pentabiotic (Zoetis)
- Ketoprofen (Ceva)
- Sterile water for injection (Equiplex)
- Povidone-iodine alcoholic solution (Aurora Pharmaceutical, catalog number: 19004)
Equipment
- Molt elevator (Roboz, catalog number: RS-8830)
- Needle holder (Fine Science Tools, catalog number: 12010-14)
- Analytical scale (Marte, model: ATX 224)
- pH meter (Marte, model: MB10)
- Small animal scale (Bonther, model: LS5)
- Pet hair clipper (Amplavet, catalog number: 950042)
- Stereotaxic apparatus (Kopf, model: 900)
- Heating pad (PhysioSuite®, Kent Scientific, catalog number: PS-02)
- Dental drill (Bonther, model: BL 2000)
- Infusion pump (Insight Equipment, model: Bi2000, catalog number: EFF 311)
- -20 °C freezer (VWR, model: SCBMF-2020)
- Autoclave (Prismatec, model: CS)
Software
- Sigma Plot 11 or Statistica 7.0
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Moreira-Silva, D., Vizin, R. C. L., Martins, T. M. S., Ferreira, T. L., Almeida, M. C. and Carrettiero, D. C. (2019). Intracerebral Injection of Streptozotocin to Model Alzheimer Disease in Rats . Bio-protocol 9(20): e3397. DOI: 10.21769/BioProtoc.3397.
分类
神经科学 > 神经系统疾病 > 动物模型
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link