- EN - English
- CN - 中文
Simple Synthesis of Functionalized Paramagnetic Beads for Nucleic Acid Purification and Manipulation
用于核酸纯化和操作的功能化顺磁性磁珠的简单合成方法
(*contributed equally to this work) 发布: 2019年10月20日第9卷第20期 DOI: 10.21769/BioProtoc.3394 浏览次数: 11307
评审: Imre GáspárManish Kumar PatelAnonymous reviewer(s)

相关实验方案
深海无脊椎动物组织保存的比较方案:DNA/RNA Shield 与液氮在高质量核酸双重提取中的应用对比
Ana S. Gomes [...] Olivier Laroche
2025年11月20日 1319 阅读
Abstract
The purification of nucleic acids is one of the most common procedures employed in modern molecular biology laboratories. Typically, commercial column-based protocols are utilized to isolate DNA or RNA from various sources. However, these methods not only require specialized equipment, but are also extremely expensive for high-throughput applications. Although an elegant answer to this issue can be provided by paramagnetic beads, bead-based open-source protocols have been limited in the past. Here, we provide an easy to follow step-by-step manual for the synthesis of paramagnetic beads, as well as their functionalization with either a silica- or a carboxyl-surface that can be used to replace the commercial columns with self-made magnetic beads. Together with a variety of detailed protocols for their use in high-throughput nucleic acids extractions, this bead synthesis method forms the recently published open platform Bio-On-Magnetic-Beads (BOMB), which is available on PLOS Biology (Oberacker et al., 2019). Updated protocols can be found on the associated webpage (https://bomb.bio).
Keywords: Carboxyl-coating (羧基涂层)Background
The ability to achieve solid-phase reversible immobilization (SPRI) (DeAngelis et al., 1995) is one of the most useful characteristics of functionalized paramagnetic nanoparticles (MNPs). Together with their scalability and potential for automation, MNPs are ideal candidates for the development of high-throughput protocols regarding nucleic acid purification (Hawkins et al., 1994; DeAngelis et al., 1995; Rohland and Reich, 2012). The small nano- or microparticles are able to reversibly bind nucleic acids under dehydrating conditions and can be safely immobilized by a strong magnet during consecutive wash or manipulation steps. Bead-based protocols can be adapted easily for multi-well formats, allowing the processing of hundreds of samples simultaneously. Additionally, MNPs are very cost-effective as the beads can be obtained very cheaply, both commercially and self-made. Although their advantages over more established column-based methods are obvious, surprisingly little effort has been put in the development of open-source protocols featuring paramagnetic beads.
Protocols for the synthesis of ferrite nanoparticles have been published before (reviewed in Houshiar et al., 2014; Singh et al., 2014; Wu et al., 2015; Majidi et al., 2016). We adopted the commonly used co-precipitation method due to its efficient and robust performance and the fact that it does not require any specialized equipment (Choi et al., 2007). For this, a solution of FeCl2 and FeCl3 in a 1:2 molar ratio is prepared and slowly dripped into a preheated NaOH solution. This forms a black precipitate consisting of Fe3O4 particles with an approximate diameter of 5 to 20 nm as judged by electron microscopy (Figure 1A). As oxygen is known to interfere with ferrite precipitation reactions, the NaOH solution should be degassed and/or preheated to 80 °C or higher before adding the iron solution. After the synthesis reaction, the nanoparticles are washed multiple times with deionized water. We recommend starting the respective coating reaction immediately after the synthesis as the particles are prone to oxidation. However, it is possible to stabilize them using various chemicals, such as detergents, sodium oleate or polyvinylpyrrolidone (PVP) (reviewed in Singh et al., 2014; Majidi et al., 2016). Upon lyophilization, the core MNPs can be stored in an air-tight container under inert atmosphere.
Figure 1. Synthesis of BOMB paramagnetic beads. A. Equipment setup for BOMB MNP synthesis. Place a magnetic stir bar in both the water bath as well as the round bottom flask in order to avoid heat accumulation. B. Synthesized and washed paramagnetic core particles. C. Paramagnetic core particles in transmission electron microscopy (TEM). D. Silica-coated paramagnetic beads in scanning electron microscopy (SEM) and TEM. E. Carboxyl-coated paramagnetic beads in light microscopy (LM), with and without an applied magnet.
Encasing of the ferrite nanoparticles in a silica coat prevents oxidation and leakage of iron ions and provides an inert surface for nucleic acid precipitation without the risk of irreversible binding. The silica deposition reaction outlined here is based on a modified version of the Stöber method (Stöber et al., 1968). It utilizes tetraethyl orthosilicate (TEOS) which is hydrolyzed in a basic environment, leading to the deposition of a SiO2 layer surrounding the paramagnetic core. Conveniently, the thickness of the silica coat, and therefore the size of the beads, can be controlled by the amount of TEOS added to the reaction (Kim et al., 2007), allowing a precise adjustment depending on the respective downstream application. The provided standard coating protocol yields silica-coated beads with an average diameter of approximately 400 nm (Figure 1B), which perform well for a wide range of nucleic acid purification and manipulation experiments from bacteria, mammalian cells, tissues (Figure 2) and many more (Oberacker et al., 2019).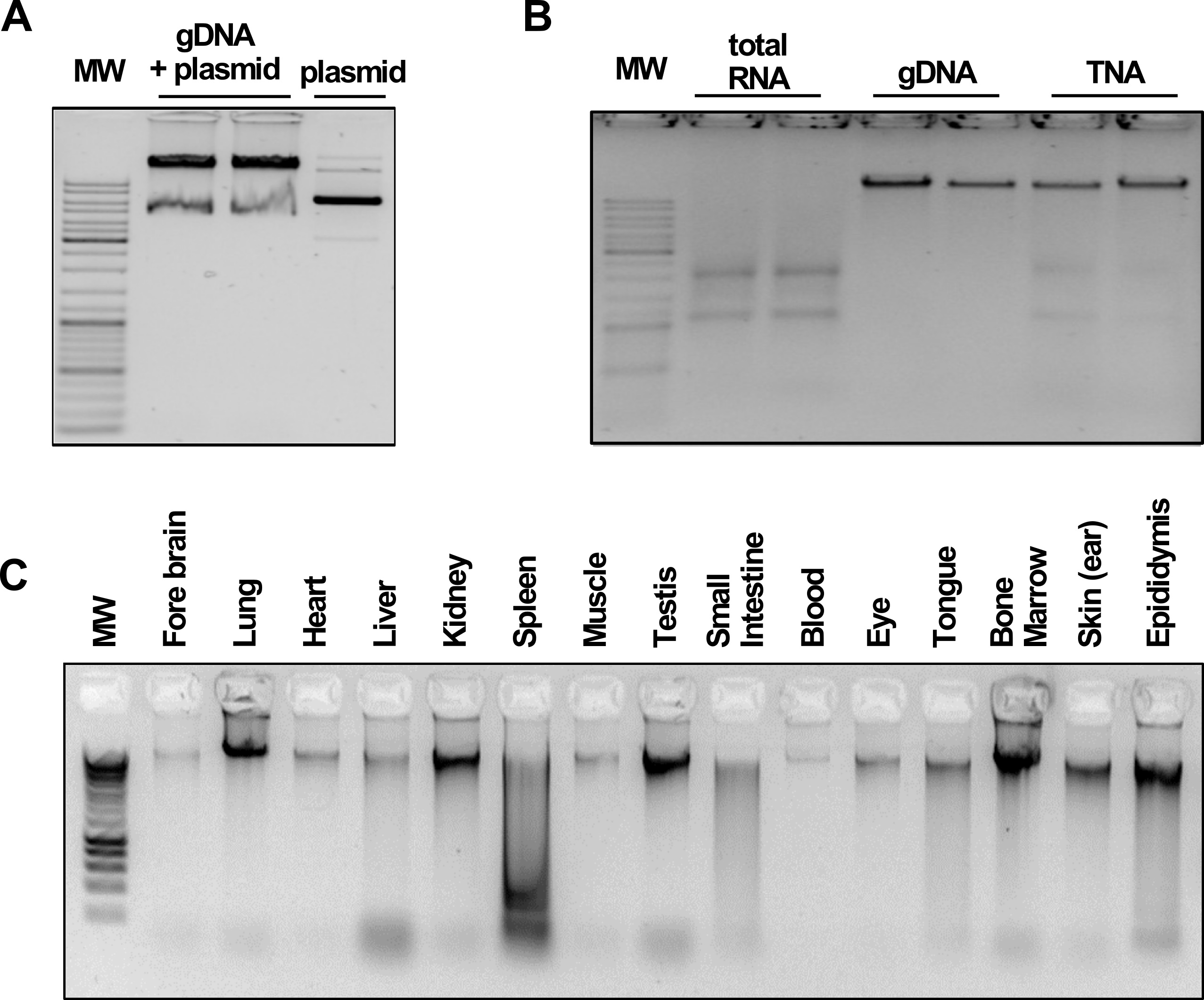
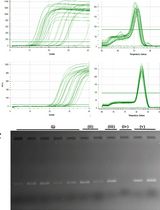
Figure 2. BOMB nucleic acid extractions. Data and figures are taken from Oberacker et al. (2019). A. Total DNA and plasmid extracted from E. coil. MW: GeneRuler DNA Ladder Mix (Thermo). B. TNA, DNA and RNA isolation from HEK293 cells. MW: GeneRuler DNA Ladder Mix (Thermo). C. gDNA isolated from various rabbit (O. cuniculus) tissues. MW: Hyperladder I (Bioline).
As an alternative to a silica-coat around the ferrite particles, a layer of a carboxyl-modified polymer can provide stability as well as a surface with a weak negative charge, thus altering the electrostatic interaction with nucleic acids and consequently the functionality of the beads (Figure 1C). Here, we use methacrylic acid (MAA) monomers, which form a layer of polymethacrylic acid (PMAA) around the ferrite core particles during a free-radical retrograde precipitation polymerization reaction (Aggarwal et al., 1996). Potentially, carboxyl- and silica-surfaces can be further modified to enable a broad range of additional functionalities to the paramagnetic beads.
Materials and Reagents
- 60 ml syringe (HENKE SASS WOLF, catalog number: 8300006680)
- 0.2 µm sterile filter (Sarstedt, catalog number: 83.1826.001)
- Protective clothing (lab coat, eye protection, gloves)
- Stand with boss head sockets and retort clamps (Bochem, catalog numbers: 5000, 5300, 5401)
- 250 ml dripping funnel (Buhler, NS29/32)
- Heat resistant bowl for a water bath
- Heat resistant 2.5 L bottle
- 0.5 or 1 L round bottom flask (Schott Duran, NS 29/32, catalog number: 2172154)
- Sterile plastic 0.5 L bottles (Corning® Costar®, Nunc® T175 uncoated PS flask)
- 50 ml conical centrifuge tube (Sarstedt, catalog number: 62.547.004)
- Ammonia solution (25% NH4OH) (EMD Millipore, catalog number: 1.05432)
- Ethanol (99% C2H6O) (Riedel-de Haёn, catalog number: 34963) or lower grade
- Hydrochloric acid (37% HCl fuming) (Roth Chemicals, catalog number: 4625.1)
- Iron(II) chloride 4-hydrate (≥ 99% FeCl2•4H2O) (Honeywell/Fluka, catalog number: 44939)
- Iron(III) chloride (≥ 98.5% FeCl3) (Roth Chemicals, catalog number: 5192.3)
- Methacrylic acid (≥ 99% C4H6O2) (Merck, catalog number: 155721)
- Potassium persulfate (≥ 99% K2S2O8) (Merck, catalog number: 216224)
- Sodium dodecyl sulfate (NaC12H25SO4) (Merck, catalog number: L3771-100G)
- Sodium hydroxide (≥ 99% NaOH) (Roth Chemicals, catalog number: 6771.1)
- Tetraethyl orthosilicate (≥ 99% Si(OC2H5)4) (Merck, catalog number: 86578)
- 2 M NaOH (see Recipes)
- 0.1 M HCl (see Recipes)
- Fe2/3 solution (see Recipes)
Note: Please consult appropriate MSDS information before working with these chemicals! Use a lab coat, gloves and eye protection at all times! The chemicals are available from other providers as well. No preference is given to the indicated vendors.
Equipment
- Fume hood
- Vacuum filtration systems (Corning, catalog number: UD-29530-02)
- Magnetic stirrer with heating (Heidolph, catalog number: 504-10000-00)
- Magnetic stir bars
- Strong neodymium permanent magnet (e.g., Neodymium disc magnet 80 x 10 mm grade N45 adhesive force ~300 kg–be extremely careful when using such strong magnets!)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Oberacker, P., Stepper, P., Bond, D., Hipp, K., Hore, T. A. and Jurkowski, T. P. (2019). Simple Synthesis of Functionalized Paramagnetic Beads for Nucleic Acid Purification and Manipulation. Bio-protocol 9(20): e3394. DOI: 10.21769/BioProtoc.3394.
分类
分子生物学 > DNA > DNA 提取
分子生物学 > RNA > RNA 提取
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link