- EN - English
- CN - 中文
Cryo-transmission Electron Microscopy of Outer-inner Membrane Vesicles Naturally Secreted by Gram-negative Pathogenic Bacteria
革兰阴性致病菌自然分泌的外-内膜囊泡低温透射电子显微镜观察
发布: 2019年09月20日第9卷第18期 DOI: 10.21769/BioProtoc.3367 浏览次数: 6737
评审: Alba BlesaKristin L. ShinglerBeatrice Li
Abstract
A protocol was developed to visualize and analyze the structure of membrane vesicles (MVs) from Gram-negative bacteria. It is now accepted that these micrometric spherical vesicles are commonly produced by cells from all three domains of life, so the protocol could be useful in the study of vesicles produced by eukaryotes and archaea as well as bacteria. The multiplicity of functions performed by MVs, related to cell communication, interaction with the immune system, pathogenesis, and nutrient acquisition, among others, has made MVs a hot topic of research.
Due to their small size (25-300 nm), the observation of MVs requires electron microscopy and is usually performed by transmission electron microscopy (TEM) of negatively stained MVs. Other protocols applied for their visualization include scanning electron microscopy, TEM after fixation and embedding of vesicles, or even atomic force microscopy. In some of these techniques, vesicle structure is altered by drying, while others are time-consuming and most of them can generate artifacts. Cryo-TEM after plunge freezing allows the visualization of samples embedded in a thin film of vitreous ice, which preserves their native cellular structures and provides the highest available resolution for the imaging. This is achieved by very high cooling rates that turn the intrinsic water of cells into vitreous ice, avoiding crystal formation and phase segregation between water and solutes. In addition to other types of characterization, an accurate knowledge of MV structure, which can be obtained by this protocol, is essential for MV application in different fields.
Background
In recent years, many studies have been conducted on membrane vesicles (MVs) produced by Gram-negative bacteria (Kulp and Kuehn, 2010). MVs are spherical membranous structures with diameters ranging between 20 and 300 nm, and they enable a protected secretion of proteins, lipids, RNA, DNA and other effector molecules (Beveridge, 1999; Mashburn-Warren and Whiteley, 2006; Ellis and Kuehn, 2010). The ability of bacterial MVs to interact with and enter host cells has prompted their exploration for novel clinical and biotechnological applications (Chen et al., 2010; Gujrati et al., 2014; Robbins and Morelli, 2014; van der Pol et al., 2015).
Different methods have been used to characterize and quantify bacterial MVs such as protein or lipopolysaccharide quantification, nanoparticle tracking analysis, flow-cytometry or proteomic analysis among others, but none of them allow MV visualization or clarify MV structure (Kulp and Kuehn, 2010). In many studies, MV-producing cells and vesicle morphology have been visualized using conventional electron microscopy techniques such as scanning electron microscopy or thin-section transmission electron microscopy (TEM) of samples chemically fixed and dehydrated at room temperature (Fiocca et al., 1999; McBroom and Kuehn, 2007; Deatherage et al., 2009; Alves et al., 2015; Ercoli et al., 2015). However, in both methods the extracellular matter in which MVs are found has a propensity to collapse and be removed during sample preparation (Beveridge, 1999; Fiocca et al., 1999; Nevot et al., 2006; Mashburn-Warren and Whiteley, 2006; McBroom and Kuehn, 2007; Deatherage et al., 2009; Ellis and Kuehn, 2010; Chen et al., 2010; Gujrati et al., 2014; Robbins and Morelli, 2014; Alves et al., 2015; Ercoli et al., 2015; van der Pol et al., 2015). TEM observation of negatively stained MVs is the most commonly used technique to assess MV presence and morphology. In this case, MVs are dried on a grid at room temperature and most of them are visualized as irregular and wrinkled round structures (Figure 1A). Although useful for confirming the presence of MVs, this technique does not provide enough resolution to visualize their exact shape or the presence of atypical MVs or artifacts, which may arise from the preparation process or when working with new or genetically manipulated strains (Bernadac et al., 1998; Kolling and Matthews, 1999; Wai et al., 2003; Lee et al., 2007; McBroom and Kuehn, 2007; Roier et al., 2016). The variability created by these features can hamper studies evaluating the application of MVs in different fields (Gujrati et al., 2014; van der Pol et al., 2015). Furthermore, in conventional electron microscopy techniques, samples have to be observed under a high vacuum and the electron beam leads to the destruction of native biological structure (Nevot et al., 2006).
In cryo-transmission electron microscopy (cryo-TEM), biological specimens are immobilized or fixed using physical procedures based on freezing. The goal is to obtain vitreous or amorphous ice from the sample water after ultra-rapid freezing, avoiding ice crystal formation and thus preserving the cellular structures. In contrast with conventional methods, ultra-rapid freezing immobilizes all molecules in a sample within milliseconds. After this instantaneous cryo-immobilization, it is very important to maintain this state throughout the imaging acquisition in the cryo-electron microscope (Cavalier et al., 2008).
In recent years, improvements in cryo-TEM techniques have enabled the imaging of biological specimens with greatly enhanced resolution and close to their native state, which has refined our knowledge of bacterial structures, including bacterial MVs (Renelli et al., 2004; Jensen and Briegel, 2007; Studer et al., 2008; Palsdottir et al., 2009; Frias et al., 2010; Perez-Cruz et al., 2013 and 2015).
There are several methods for freezing samples. Plunging samples into a pre-cooled cryogen, such as ethane or propane, is a common technique for freezing suspensions (viruses, liposomes, bicelles, micelles...), molecular assemblies (proteins, nucleic acids...), isolated organelles and cell structures and even small cells such as bacteria (Dobro et al., 2010). This method, known as plunge freezing, has been used to visualize bacterial MVs (Renelli et al., 2004), and has revealed, for example, the existence of a new type of Gram-negative MVs produced by environmental and pathogenic bacteria, named outer-inner membrane vesicles (O-IMVs) (Figures 1B, 2C and 2) (Perez-Cruz et al., 2013 and 2015).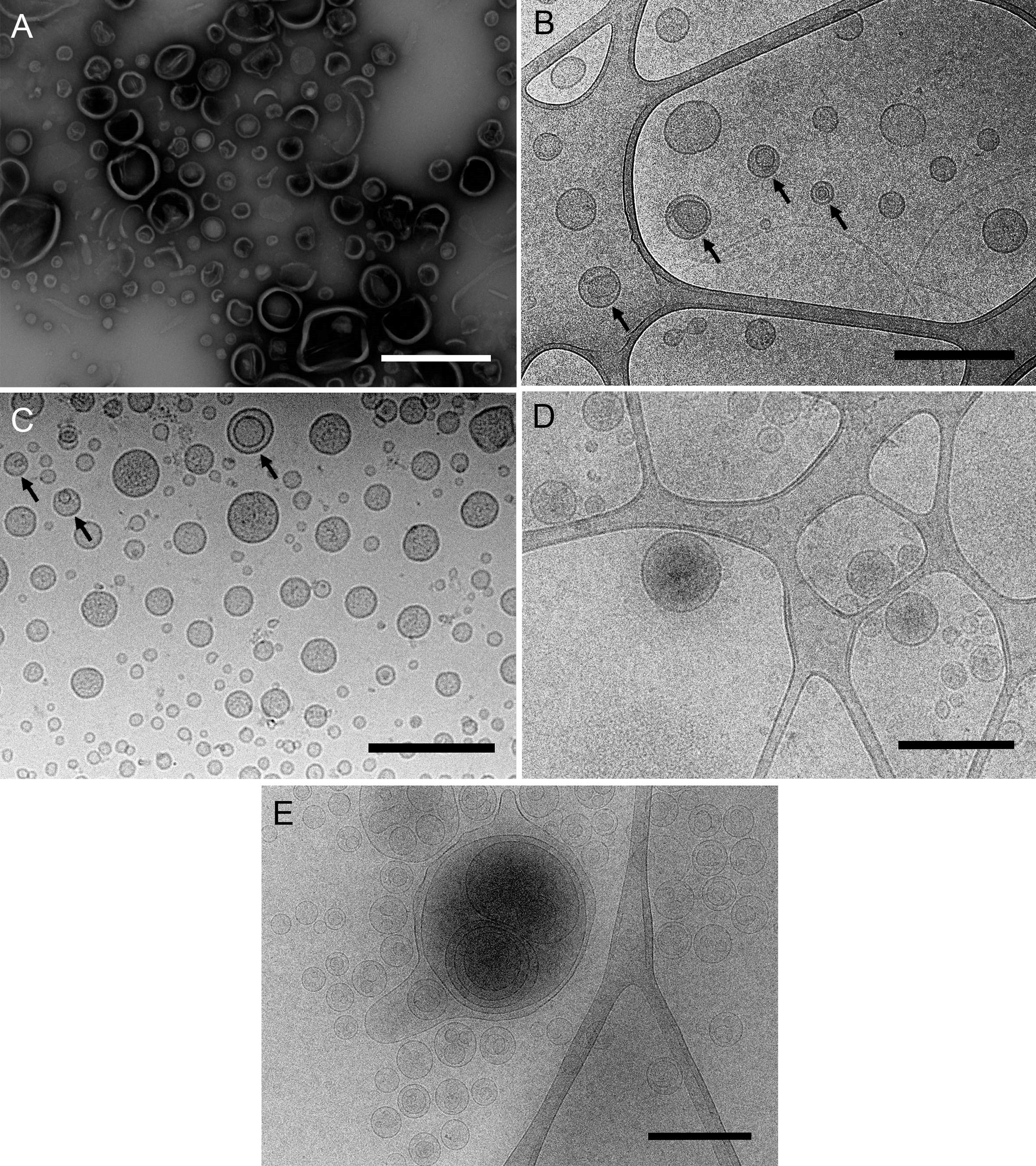
Figure 1. Different samples of isolated MVs observed by (cryo-)TEM. A. MVs from Shewanella vesiculosa M7T prepared by negative staining and observed by TEM. Vesicles appear collapsed and as irregular and wrinkled round structures. B and C. MVs from Shewanella vesiculosa M7T and (C) Acinetobacter baumannii AB41 prepared by plunge freezing and observed by cryo-TEM. MVs appear as regular round structures and different types can be observed: conventional bacterial outer membrane vesicles with one membrane layer and a new type called outer-inner membrane vesicles (O-IMV) with two membrane layers (black arrows). D and E. Exosomes produced by melanoma cancer cells and (E) liposomes synthesized from lipids. All types of vesicles appear as regular round structures and membrane layers are clearly visualized. Scale bars = 500 μm.
Plunge freezing of MVs allows us to observe their exact shape, size and integrity, the presence of one or more membrane layers, and attached surface-associated structures like viruses, among other features (Perez-Cruz et al., 2016).
Bacterial MVs are not the only focus of investigation. Another expanding research area is the study of extracellular vesicles (EVs), which are released by cells from all domains of life-Eukarya, Archaea and Bacteria-and are considered intercellular communicasomes, acting as a mechanism for distance delivery of active compounds between cells (Yoon et al., 2014). EVs have demonstrated great promise as natural drug delivery systems loaded with therapeutic molecules (Armstrong and Stevens, 2018). Other candidates for the improvement of drug delivery systems are liposomes and other lipidic formulations (Alavi et al., 2017), varying in size and lamellar number, and cryo-TEM observation could also be applied to advance their development. The described protocol therefore has potential application for other types of vesicles currently under active research (Figures 1D and 1E).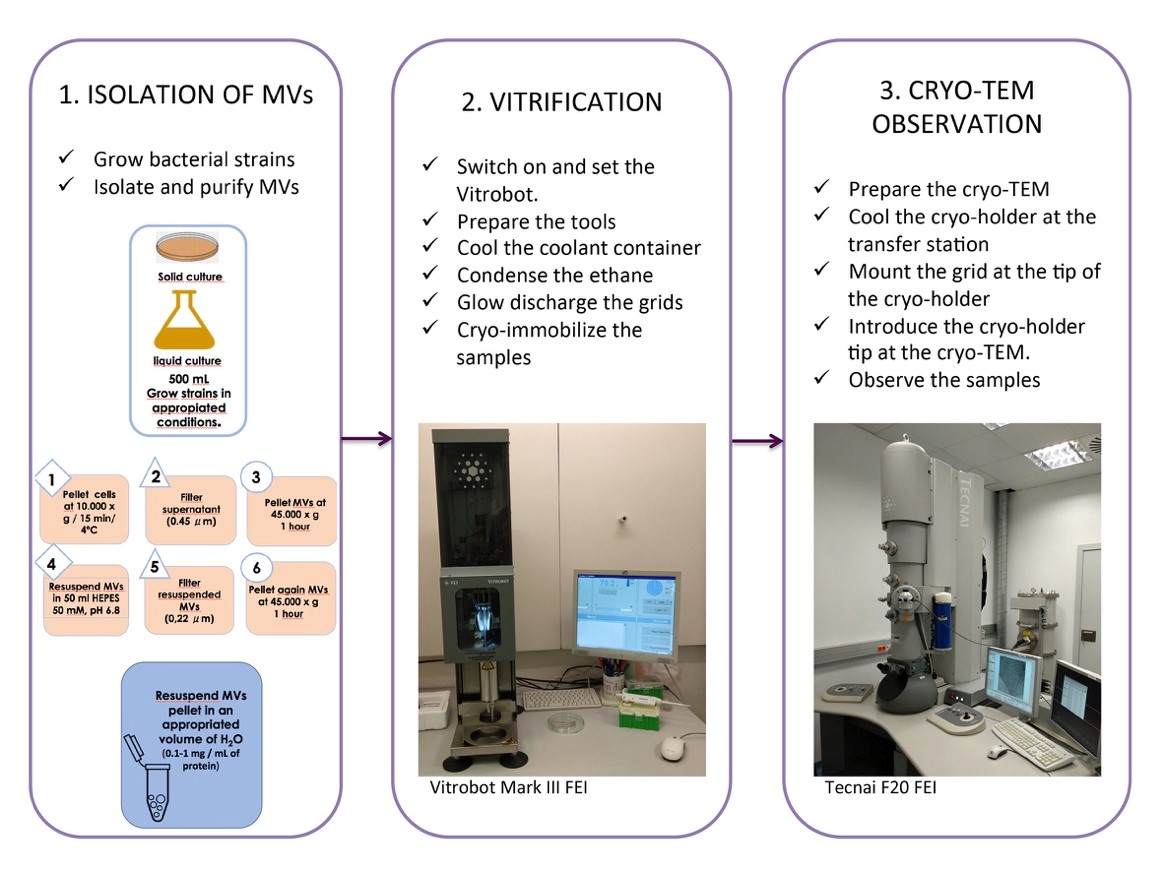
Figure 2. Cryo-TEM of isolated MVs, a general overview of the whole protocol
Materials and Reagents
- Tweezers No. 5 (Dumont, catalog number: 21974-1)
- Safe tweezers (Wiha, catalog number: 44518)
- Vitrobot forceps (Ted Pella, catalog number: 47000-500)
- Micropipettes 20, 100 and 1000 (Eppendorf, model: Research)
- Membrane filters, white, individually packed. 0.45 µm, diameter 47 mm, sterile (ME 25 ST, Whatman®, catalog number: 10401670)
- Disposable filter device, 0.22 µm, sterile and non-pyrogenic polyethersulfone membrane with polypropylene housing (PURADISC 25 AS, Whatman®, catalog number: 6780-2502)
- 250 ml centrifuge polypropylene bottles (Beckman Coulter, catalog number: 356011)
- 50 ml centrifuge polypropylene tubes (Nalgene, catalog number 3139-0050)
- 500 ml and 1000 ml screw neck glass bottles (DURAN®)
- Vacuum filtration unit (DURAN® , catalog number: XT09.1)
- Chocolate agar plates (Beckton Dickinson, catalog number: PA-254035.05)
- Lacey Carbon 300 mesh grids (Ted Pella, catalog number: 01895-F)
- Quantifoil® R 2/2 Cu 200 mesh grids (Quantifoil Micro Tools GmbH, catalog number: Q23209)
- Holey Carbon 300 mesh grids (Agar, catalog number: AGS147-3)
- Filter paper for Vitrobot (Ted Pella, catalog number: 47000-100)
- Cryo Grid Box base only (Ted Pella, catalog number: 160-41)
- Cryo Grid Box Handling Rod (Ted Pella catalog number: 160-46)
- Neoprene gloves (Seton, catalog number: 10STA002)
- Parafilm (Sigma-Aldrich, catalog number: P7793-1EA)
- Petri dish (Fisher Scientific, catalog number: 11812532)
- Yellow pipette tips (Sigma-Aldrich, catalog number: EP4925000111)
- Blue pipette tips (Sigma-Aldrich, catalog number: EP3124000121)
- Neisseria gonorrhoeae (ATCC 43069)
- Pseudomonas aeruginosa PAO1 (Own collection)
- Acinetobacter baumannii AB41 (Clinical isolate)
- Ringer ¼ (Sigma-Aldrich, catalog number: 96724-100TAB), prepare the solution as per the manufacturer’s instructions (Reference 1)
- Trypticase soy broth (Oxoid, catalog number: CM129), prepare the solution as per the manufacturer’s instructions (Reference 10)
- Müeller-Hinton broth (Oxoid, catalog number: CM0405), prepare the solution as per the manufacturer’s instructions (Reference 11)
- HEPES (Sigma-Aldrich, catalog number: 7365-45-9), prepare the solution as per the manufacturer’s instructions (Reference 18)
- Modified Lowry Protein Assay kit (Thermo Scientific, catalog number: 23240)
- Ethanol 96% (Sigma-Aldrich, catalog number: 16368)
- Ethane (AIR liquide)
- Liquid nitrogen (AIR liquide)
Equipment
- Fume hood (Flores Valles, model: LVG 190)
- Glow Discharge unit (BALTEC, model: CTA005)
- Minishaker (IKA®, model MS2)
- Vitrification Robot (FEI, model: VitrobotTM Mark III)
- Cryo-holder and cryo-transfer station (Gatan, model: 626)
- Cryo-electron microscope (FEI, model: Tecnai F20 200 kV) equipped with a CCD camera (FEI, model: Eagle 4kx4k)
- Orbital shaker (Innova® 44, Incubator Shaker Series, New Brunswick Scientific)
- Centrifuge (Beckman Coulter, model: Avanti J-20 XP)
- Centrifuge (Beckman Coulter, model: Allegra 25R)
Software
- Vitrobot V1.05B051 (FEI)
- Tecnai version 4.3 (FEI)
- TEM Imaging & Analysis version 4.4 (FEI)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Delgado, L., Baeza, N., Pérez-Cruz, C., López-Iglesias, C. and Mercade, E. (2019). Cryo-transmission Electron Microscopy of Outer-inner Membrane Vesicles Naturally Secreted by Gram-negative Pathogenic Bacteria. Bio-protocol 9(18): e3367. DOI: 10.21769/BioProtoc.3367.
分类
微生物学 > 微生物细胞生物学 > 细胞成像
细胞生物学 > 细胞成像 > 电子显微镜
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link