- EN - English
- CN - 中文
A Robust, One-step FRET Assay for Human Heparanase
人乙酰肝素酶的一步快速FRET检测
发布: 2019年09月05日第9卷第17期 DOI: 10.21769/BioProtoc.3356 浏览次数: 4658
评审: Vivien Jane Coulson-ThomasPaul Ross Wildermantarsis ferreira

相关实验方案
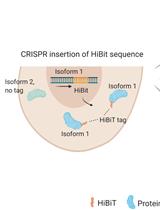
通过CRISPR-Cas9介导的HiBiT标签对高度同源蛋白水平进行异构体特异性半定量测定
Kristina Seiler [...] Mario P. Tschan
2023年07月20日 2519 阅读
Abstract
Heparanase, an endo-β-D-glucuronidase, cleaves cell surface and extracellular matrix heparan sulfate (HS) chains at distinct sites and plays important biological roles including modulation of cell growth and metastasis. Although a number of different types of heparanase assays have been reported to date, most are labor intensive, complex and/or expensive to carry out. We reasoned that a simpler heparanase assay could be developed using heparin labeled with Dabcyl and EDANS as donor and acceptor fluorophores so as to generate a FRET signal. Our results show that a more robust heparanase assay could be developed based on the principle studied herein and more homogeneous preparation of heparin. Yet, the assay in its current form could be used for routine screening of potential inhibitors in a high-throughput manner as well as for studying heparanase activity expressed in tumors as well as biological fluids like plasma.
Keywords: Heparanase (乙酰肝素酶)Background
Human heparanase is an endo-β-D-glucuronidase that cleaves heparan sulfate (HS) chains present in proteoglycan form on cell surfaces and in the extracellular matrix (Fairbanks et al., 1999; Kussie et al., 1999; Toyoshima and Nakajima, 1999; Dempsey et al., 2000; Sanderson et al., 2017). Although other sequences are also likely to be targeted by heparanase (Peterson and Liu, 2013), substrate specificity studies indicate that heparanase preferably cleaves the 1→4-inter-glycosidic bond between a glucuronic acid (GlcA) and glucosamine-N,6-disulfate (GlcNS6S) residues.
The importance of heparanase as a therapeutic target has led to the development of several biochemical and biophysical assays over the past 2 decades. Despite the availability of numerous assays, no particular assay appears to have been broadly used to understand heparanase biology, pharmacology, and drug discovery. The reported assays typically suffer from the involvement of multiple steps and incubation up to 24 h (Freeman and Parish, 1997; Behzad and Brenchley, 2003; Tsuchida et al., 2004; Enomoto et al., 2006; Hammond et al., 2010; Schiemann et al., 2012; Melo et al., 2015). An easier, one-step assay would greatly help deduce inhibitors, understand substrate specificity, elucidate the mechanism of action, and clarify enzymatic or non-enzymatic role in cellular systems. A more robust heparanase assay would be easier to implement (fewer steps, faster screening time, no immobilization, no post-assay signal development), which is adaptable to microplate format, enable assaying active heparanase in cellular media, and perhaps help monitor heparanase in vivo. We hypothesized fluorescence resonance energy transfer (FRET)-based assay may offer a one-step solution that can address several attributes. This documents, method of developing a FRET-based assay for active heparanase. Based on our work, FRET-enabled heparin chain could effectively serve as a substrate of heparanase, help detect an active enzyme in media, and to screen potential inhibitors.
Materials and Reagents
- 1 cm path length quartz cuvette (Hellma®, catalog number: Z801712)
- 96-well plates (Corning, catalog number: 3897)
- Wilmad-LabGlassTM NMR Tubes (Fisher Scientific, 16-800-554)
- 6-well tissue culture-treated plates (Cyto one, catalog number: CC7672-7506)
- Low-Retention Microcentrifuge Tubes (Fisher Scientific, catalog number: 02-681-331; Amber, catalog number: 05-402-31)
- FisherbrandTM Micro Stir Bars (Fisher Scientific, catalog number: 16800523)
- FLEX-COLUMN® Economy Columns (Fisher Scientific, catalog number: K420400-1030)
- SpectrumTM Spectra/PorTM Float-A-LyzerTM G2 Dialysis Devices (Fisher Scientific, catalog number: 08-607-020)
- Fisher brandTM Class B Amber Glass Threaded Vials with Attached Caps (Fisher Scientific, catalog number: 14-955-332)
- PierceTM Protein Concentrator PES, 3K MWCO, 2-6 ml (Fisher Scientific, catalog number: PI88515)
- Sephadex G-15 (GE Healthcare, catalog number: 17002001)
- MCF7 (ATCC® HTB-22TM) and HEK 293T (ATCC® ACS-4500TM) cell lines
- Heparin sodium salt from porcine intestinal mucosa (Sigma-Aldrich, catalog number: H4784-1G stored at 4 °C)
- Dabcyl C2 amine (100 mg) (AnaSpec, catalog number: AS-81819, stored at -20 °C under N2)
- EDANS, sodium salt, 5- [(2-aminoethyl) amino] naphthalene-1-sulfonic acid, sodium salt (0.5 g) (AnaSpec, catalog number: AS-23886, stored at -20 °C under N2)
- N-(3-Dimethylaminopropyl)-N′-ethyl carbodiimide hydrochloride (EDC) (Sigma-Aldrich, catalog number: 03449, stored at -20 °C)
- N-hydroxysuccinimide (NHS) (Sigma-Aldrich, catalog number: 130672-5G, -20 °C under N2 )
- Glacial Acetic Acid (HPLC grade) (Fisher Scientific, catalog number: A35-500)
- Methyl sulfoxide, 99.7%, Extra Dry, anhydrous, SC (AcroSealTM, ACROS OrganicsTM, catalog number: AC610971000)
- Thermo ScientificTM NERLTM High Purity Water (Fisher Scientific, catalog number: 23-249-590)
- Water (HPLC grade) (Fisher Scientific, Fisher Chemical W5-4)
- Suramin (Sigma-Aldrich, catalog number: S2671)
- Deuterium oxide, D2O (Sigma-Aldrich, catalog number: 151882)
- Sodium acetate (Sigma-Aldrich, catalog number: S7545)
- Sodium Chloride (Fisher Scientific, catalog number: BP358-1)
- HEPES (Fine White Crystals/Molecular Biology) (Fisher BioReagents, catalog number: BP310-500)
- HyCloneTM Fetal Bovine Serum (FBS) (Fisher BioReagents, catalog number: SH3007102)
- Dulbecco's Modified Eagle Medium (DMEM) growth media (Invitrogen, USA)
- (Optional) Heparanase protein (R&D systems, catalog number: 7570-GH-005)
- Antibiotic-antimycotic Solution (AA) (Fisher Scientific, catalog number: 15-240-062), antibiotic spectrum: Amphotericin B, Penicillin, Streptomycin
- Assay buffer (see Recipes)
Equipment
- FreeZone Plus 6 Lyophilizer (Labconco Corporation)
- Peristaltic Pump P-1 (GE healthcare, catalog number: 18111091 )
- Temperature controlled Centrifuge (Beckman coulter, model: Microfuge 20R; Eppendorf, model: 5804R)
- Flex Station III (Molecular Devices, CA)
- Shimadzu Spectrophotometer (Shimadzu Scientific Instruments, USA)
- Fluorimeter PTI QM-400 (Horiba Canada)
- pH meter (Denver Instrument, model: 220)
- 400 MHZ topspin H1NMR Bruker
- C24 classic series Incubator (New Brunswick Scientific, NJ)
- Precision Water bath (Precision Scientific, model: 25)
- -20 °C (Norlake Scientific) and -80 °C freezer (Thermo scientific)
- Forma Series II, water jacketed CO2 incubator (Thermo Electron Corporation, USA)
- Stirring Hotplate (FisherbrandTM IsotempTM, catalogue number: SP88850200)
- Fluorescence cuvette (Hellma®, catalog number: 101.015-QS; Cell Holder, catalog number: 013.013)
Software
- SigmaPlot 13 (Systat Software, Inc. or OriginPro or Kaleidagraph)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Sistla, J. and Desai, U. R. (2019). A Robust, One-step FRET Assay for Human Heparanase. Bio-protocol 9(17): e3356. DOI: 10.21769/BioProtoc.3356.
分类
癌症生物学 > 通用技术 > 生物化学试验
生物化学 > 糖类 > 寡糖 > 标记
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










![N-连接寡糖的[2-<sup>3</sup>H] 甘露糖标记和分析](https://en-cdn.bio-protocol.org/imageup/arcimg/20170718074630401.jpg?t=1770161446)