- EN - English
- CN - 中文
A High-throughput and Pathophysiologically Relevant Astrocyte-motor Neuron Co-culture Assay for Amyotrophic Lateral Sclerosis Therapeutic Discovery
高通量病理生理相关星形胶质细胞运动神经元共培养技术在肌萎缩侧索硬化治疗中的应用
发布: 2019年09月05日第9卷第17期 DOI: 10.21769/BioProtoc.3353 浏览次数: 7044
评审: Xi FengSunanda MarellaAnonymous reviewer(s)

相关实验方案
使用基于纤维蛋白的生物墨水对间充质干细胞衍生的神经组织进行3D生物打印的方案
Milena Restan Perez [...] Stephanie Michelle Willerth
2023年05月05日 2865 阅读
Abstract
Amyotrophic lateral sclerosis (ALS) is an adult onset neurological disorder characterized by loss of motor neurons leading to progressive muscle wasting and eventually death. Astrocytes play a key role in disease pathogenesis. However, the ability to study astrocytic support towards motor neurons in ALS has been limited by a lack of sustainable high-throughput human cell models. Moreover, the ability to assess how astrocytic support of motor neurons is influenced by drug treatment or nutritional supplementation has been hampered by the lack of robust methodology. We have developed a high-throughput astrocyte motor neuron co-culture assay, which, by using Hb9-GFP+ motor neurons enables researchers to assess how ALS affects the ability of astrocytes to support motor neurons in 384-well plates. Moreover, astrocyte function can be manipulated by nutritional supplementation or drug treatment to identify possible therapeutic targets.
Keywords: ALS (ALS)Background
Amyotrophic lateral sclerosis (ALS) is a neurological disorder resulting in degeneration of both upper and lower motor neurons, resulting in the progressive failure of the neuromuscular system. Death typically occurs 2-3 years post-symptom onset, due to a lack of effective therapies. Although death of motor neurons is a key feature of ALS, the disease is non-cell autonomous with neighboring cells such as astrocytes, microglia and oligodendrocytes playing a key role (Ferraiuolo et al., 2011b; Ferraiuolo et al., 2016; Frakes et al., 2017; Vandoorne et al., 2018). Astrocytes play a crucial metabolic role in the CNS as they are the major source of brain glycogen, and astrocyte lactate can be taken up by motor neurons and used as a source of energy (Pellerin and Magistretti, 1994). Several mechanisms have been implicated in astrocyte-mediated motor neuron death, including release of nitric oxide and prostaglandin E2, altered glutamate transporter expression, reduced lactate release and reduced extracellular vesicle mediated release of miRNA (Lin et al., 1998; Ferraiuolo et al., 2011a; Ferraiuolo et al., 2011b; Allen et al., 2019; Varcianna et al., 2019).
Until recently, the ability to study how patient derived astrocytes affect motor neuronal function has been hampered by technological limitations. Initial pioneering studies (Haidet-Phillips et al., 2011, Re et al., 2014) demonstrated that post-mortem human sporadic and familial patient-derived astrocytes could induce motor neuron death in vitro. Although elegant, this methodology presents with limitations in terms of limited availability, scalability and represents end stage of disease. The development of techniques to reprogram fibroblasts into pluripotent stem cells (iPSCs) by Yamanaka and colleagues (Takahashi and Yamanaka, 2006) has radically improved our ability to study human CNS disorders in vitro, for a recent review see (Myszczynska and Ferraiuolo, 2016). This technological advancement was followed up by methodologies to directly reprogram mouse fibroblasts into induced neuronal progenitor cells (iNPCs) and concomitantly by Meyer and Ferraiuolo who developed novel methodologies to reprogram adult human fibroblasts into iNPCs from control and ALS subjects (Kim et al., 2011; Meyer et al., 2014). This advancement in direct reprogramming, has overcome the challenge of phenotypic inconsistency caused by clonal variation in iPSCs and has significantly shortened the reprogramming timescales, reducing costs and increasing throughput (Myszczynska and Ferraiuolo, 2016). Furthermore, an additional beneficial feature of the direct reprogramming approach is that the epigenetic state of the cell is not reset, so any aging phenotype inherent in the donor fibroblasts should be retained (Mertens et al., 2015). This subsequent development has enabled ALS researchers to assess the effect of disease on patient derived cells in vitro, reducing the reliance on post-mortem tissue and animal models of disease that may lack translational efficacy.
In the co-culture approach described in this paper, iNPCs were derived from ALS patient fibroblasts and healthy donor control fibroblasts as described previously (Meyer et al., 2014). iNPCs were differentiated into iAstrocytes by culturing in iAstrocyte media for at least 5 days (Figure 1). Mouse motor neurons expressing the green fluorescent protein (GFP) under the Hb9 motor neuron-specific promoter (called from now on Hb9-GFP+ MN) were differentiated from murine embryonic stem cells (mESCs) via embryonic bodies (mEBs), as previously described (Wichterle et al., 2002; Haidet-Phillips et al., 2011). The advantage of this approach over existing co-culture methods using post-mortem material or iPSCs, is that iAstrocyte support of motor neurons can be measured in a high-throughput, cost-effective fashion, under a number of conditions including drug treatment or nutritional supplementation. Furthermore, traditional cell-based high throughput screening methodologies that have been utilized in the ALS field have focused on animal or human cell models of the disease in monoculture, for a recent review see (McGown and Stopford, 2018). In the methodological approach adopted here, iAstrocytes can be reproducibly differentiated within a week from iNPCs and their effect on motor neuron survival can be measured in co-culture. Survival can be assessed by monitoring fluorescence of the motor neurons over time and counting the number of viable motor neurons (Meyer et al., 2014; Hautbergue et al., 2017; Allen et al., 2019). Moreover, the effect of treated or untreated iAstrocyte conditioned media on motor neuron survival can also be assessed (Varcianna et al., 2019). Therefore, this approach has the advantage over traditional high throughput monoculture methodologies of being more physiologically representative of the in vivo state and therefore has greater translational potential.
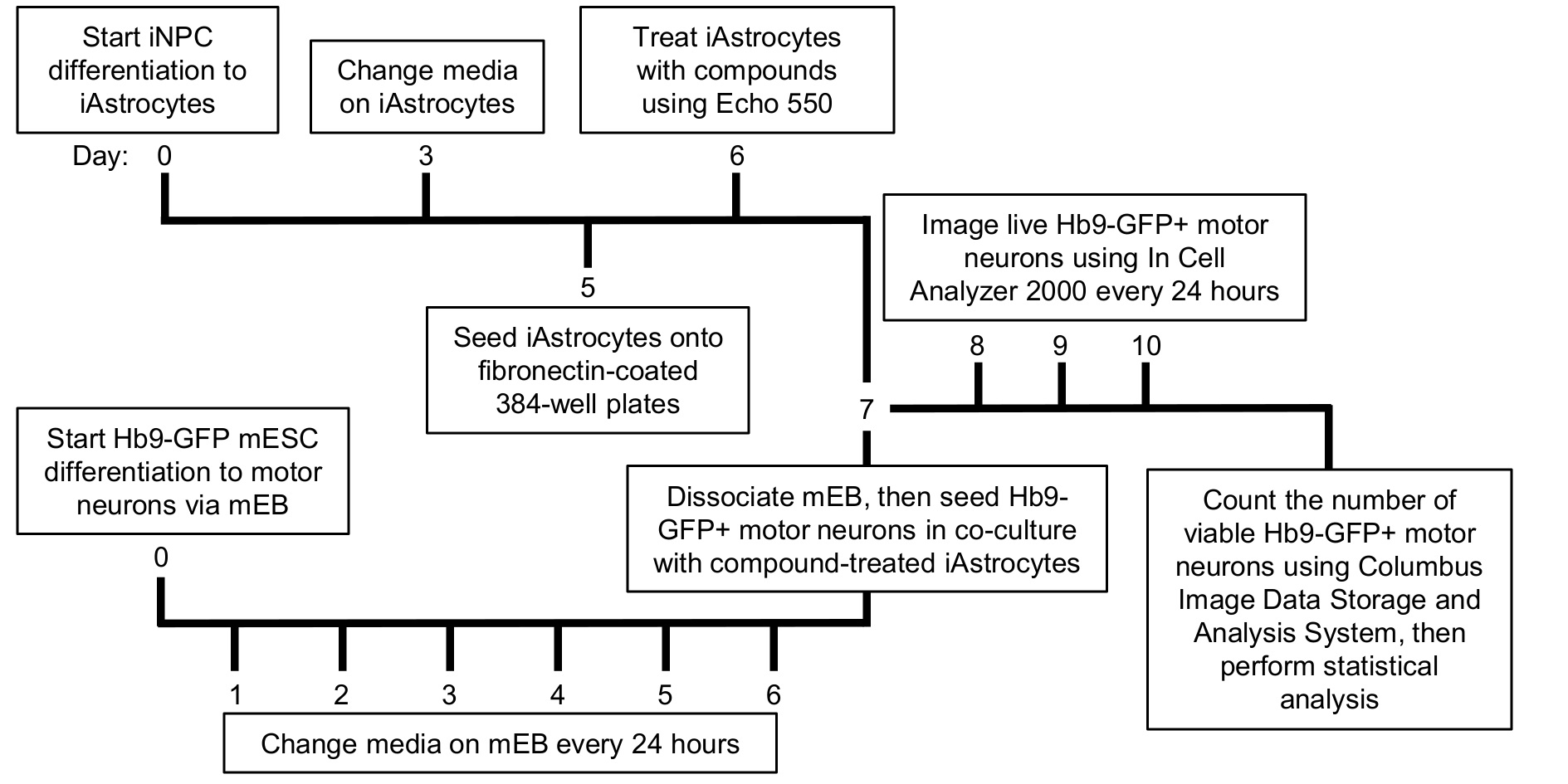
Figure 1. Timeline of the high-throughput human iAstrocyte–Hb9-GFP+ mouse motor neuron co-culture assay
Materials and Reagents
- Materials
- 10 cm tissue culture plates (Thermo Scientific, catalog number: 150350)
- 9 cm Petri dish (Scientific Laboratory Supplies, catalog number: PET2000)
- 384-well plates (Greiner-Bio, catalog number: 781091)
- 384-well PP source plates (Labcyte, catalog number: PP-0200)
- 5 ml Disposable Pipets (Fisher Scientific, catalog number: 13-676-10H)
- 10 ml Disposable Pipets (Fisher Scientific, catalog number: 13-676-10J)
- 25 ml Disposable Pipets (Fisher Scientific, catalog number: 13-678-11)
- 10 µl Pipet Tips (Fisher Scientific, catalog number: 02-707-441)
- 200 µl Pipet Tips (Fisher Scientific, catalog number: 02-707-422)
- 1,000 µl Pipet Tips (Fisher Scientific, catalog number: 02-707-402)
- 15 ml Falcon Tube (Greiner-Bio, catalog number: 188271)
- 50 ml Falcon Tube (Greiner-Bio, catalog number: 227261)
- 0.2 µm Syringe Filter (Sartorius, catalog number: 16534K)
- DMSO resistant foil lids (Brooks life sciences, catalog number: 4ti-0512)
- Steritop filter (Millipore, catalog number: S2GPT05RE)
- Sterile cell strainer, 40 µm mesh (Fisher Scientific, catalog number: 11587522)
- Sterile cell strainer, 70 µm mesh (Corning, catalog number: 431751)
- Reagents
- Primary Mouse Embryonic Fibroblasts (Sigma-Aldrich, catalog number: PMEF-CF)
- Pen-Strep (Lonza, catalog number: DE17-603E)
- 10x Trypsin (Lonza, catalog number: BE02-007E)
- 2-Mercaptoethanol (Sigma-Aldrich, catalog number: M3148)
- Accutase (Gibco, catalog number: 11599686)
- B-27 (Gibco, catalog number: 11530536)
- BDNF (Peprotec, catalog number: 450-02)
- CaCl (Sigma-Aldrich, catalog number: C1016)
- CNTF (Peprotec, catalog number: 450-13)
- D-(+)-Glucose (Sigma-Aldrich, catalog number: G7021)
- DMEM (Lonza, catalog number: 12-741F)
- DMEM/F-12, GlutaMAX (Gibco, catalog number: 11524436)
- DMSO, anhydrous (Sigma-Aldrich, catalog number: 276855)
- DNase I (Applichem Lifescience, catalog number: A3778)
- EDTA (Thermo Scientific, catalog number: 17892)
- ESC FBS (Gibco, catalog number: 11500526)
- FBS (Biosera, catalog number: FB-1090)
- FGF (Peprotec, catalogue number: 100-18B)
- GDNF (Peprotec, catalog number: 450-10)
- Ham’s F-12 Nutrient Mix (Gibco, catalog number: 15172529)
- Human Fibronectin (Sigma-Aldrich, catalog number: FC010-10MG)
- KCl (Sigma-Aldrich, catalog number: P5405)
- Knockout DMEM (Gibco, catalog number: 10389172)
- KnockOut Serum Replacement (Gibco, catalog number: 11520366)
- L-Cysteine (Sigma-Aldrich, catalog number: C7352)
- L-glutamine (Lonza, catalog number: BE-17-605E)
- MgSO4 (Sigma-Aldrich, catalog number: M2643)
- Mouse LIF (Sigma-Aldrich, catalog number: ESG1106)
- N-2 (Gibco, catalog number: 11520536)
- NaCl (Fisher Scientific, catalog number: 11904061)
- NaH2PO4 (Sigma-Aldrich, catalog number: S3139)
- NaHCO3 (Sigma-Aldrich, catalog number: S5761)
- Nitrogen (oxygen free) (BOC, catalog number: 44-Y)
- Non-Essential Amino Acids (Gibco, catalog number: 12084947)
- Papain (Sigma-Aldrich, catalog number: P4762)
- PBS Tablets (Thermo Scientific, catalog number: BR0014G)
- Retinoic Acid (Sigma-Aldrich, catalog number: R2625)
- SAG (Sigma-Aldrich, catalog number: 566660)
- Sterile 1x PBS (see Recipes)
- Sterile ultrapure water (see Recipes)
- Human iNPC Proliferation Media (see Recipes)
- Human iAstrocyte Differentiation Media (see Recipes)
- Mouse Embryonic Stem Cell (mESC) Proliferation Media (see Recipes)
- Mouse Embryonic Bodies (mEB) Differentiation Media (see Recipes)
- Mouse Motor Neuron (mMN) Media (see Recipes)
- EB Dissociation Buffer (see Recipes)
Equipment
- 4 °C fridge
- -20 °C freezer
- -80 °C freezer
- Autoclave
- Class II biosafety cabinet (NuAire, catalog number: NU-437-400E)
- CO2 Incubator (Sanyo, catalog number: MCO-19AIC)
- Echo 550 liquid handler (Labcyte, catalog number: Echo 550)
- Haemocytometer
- Harrier 15/80 benchtop centrifuge (MSE, catalog number: MSB.080.CX1.5)
- In Cell Analyzer 2000 (GE Healthcare, catalog number: 52-851714-001)
- Liquid nitrogen dewar
- Mechanical pipettes (P10, P50, P200, P1000)
- Multichannel mechanical pipettes (P10, P50)
- MultiPod Controller (Roylan Developments, catalog number: SPOD0012)
- Mechanical pipette gun
- PK120 centrifuge with T336 rota (ALC, catalog number: 11200030), and buckets for microplates (ALC, catalog number: 11210267)
- Polystyrene float for water bath
- StoragePod Enclosure (Roylan Developments, catalog number: SPOD0010)
- Water bath
- Sterile culture hood
Software
- Echo Liquid Handler Software (Labcyte)
- Echo Plate Reformat (Labcyte)
- In Cell Analyzer 2000 (GE Healthcare)
- Columbus Image Data Storage and Analysis System (PerkinElmer)
- Excel 2016 (Microsoft)
- GraphPad Prism 7.0 (GraphPad)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Stopford, M. J., Allen, S. P. and Ferraiuolo, L. (2019). A High-throughput and Pathophysiologically Relevant Astrocyte-motor Neuron Co-culture Assay for Amyotrophic Lateral Sclerosis Therapeutic Discovery. Bio-protocol 9(17): e3353. DOI: 10.21769/BioProtoc.3353.
分类
神经科学 > 基础技术 > 高通量筛选
细胞生物学 > 基于细胞的分析方法 >
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link