- EN - English
- CN - 中文
Ex vivo Drosophila Wing Imaginal Disc Culture and Furin Inhibitor Assay
果蝇翅膀成虫盘体内培养和弗林蛋白酶抑制剂检测
发布: 2019年08月20日第9卷第16期 DOI: 10.21769/BioProtoc.3336 浏览次数: 5525
评审: Abhijit KaleSveta ChakrabartiAnonymous reviewer(s)
Abstract
Furin is an evolutionarily conserved proprotein convertase (PC) family enzyme with a broad range of substrates that are essential for developmental, homeostatic, and disease pathways. Classical genetic approaches and in vitro biochemical or cell biological assays identified that precursor forms of most growth factor family proteins are processed by Furin. To quantitatively assess the potential role of Furin in cleaving and modulating intercellular dispersion of a Drosophila signaling protein, we developed a simple assay by combining genetics, ex vivo organ culture, pharmacological treatment, and imaging analyses. The protocol herein describes how to ex vivo culture Drosophila wing imaginal discs expressing a fluorescently tagged Drosophila Fibroblast Growth Factor (FGF, Branchless/Bnl) over a long period of time in the presence of Furin inhibitors and monitor the cleavage and intercellular dispersion of the truncated Bnl parts using microscopy. Although the assay described here is for assessing the effect of Furin inhibition on Bnl cleavage in the Drosophila larval wing imaginal disc, the principle and methodology can easily be adopted for any other signals, tissue systems, or organisms. This strategy and protocol provide an assay for examining Furin activity on a specific substrate by directly visualizing the spatiotemporal distribution of its truncated parts in an ex vivo-cultured organ.
Keywords: Proteolytic cleavage (蛋白水解)Background
The range of intercellular dispersion and signaling activity of many morphogenetic signaling proteins depends on 'limited proteolysis' by proprotein convertases (PCs) (LeMosy, 2006; Künnapuu et al., 2009; Wharton and Serpe, 2013; Sohr et al., 2019). PCs are serine endoprotease family enzymes that are known to cleave various growth factors including Epidermal growth factor (EGF), the Transforming Growth Factor beta/Bone Morphogenetic Protein (TGF-β/BMP) family, several Fibroblast Growth Factor (FGF) family proteins, Platelet-Derived Growth Factor (PDGF), and Vascular Endothelial Growth Factor (VEGF), all of which play a critical role in development and diseases (Khatib and Geraldine, 2006; Tulin and Stathopoulos, 2010; Rousselet et al., 2011; Künnapuu et al., 2014; Constam, 2014; Anderson and Wharton, 2017; Sohr et al., 2019). Although biochemical and cell culture assays are sufficient to demonstrate a substrate cleavage by individual PC substrate-specificity, their functional characterization in vivo is largely dependent on classical genetic approaches. However, the presence of multiple PCs in different organisms and their functional redundancy can only provide limited information about an individual PC's function. There are nine PCs in vertebrates and three in Drosophila, all expressed broadly, that share a consensus cleavage site (-Arg-X-Lys/Arg-Arg-) in their substrates. Moreover, a broad range of cellular substrates as well as extracellular matrix proteins that can also influence a signaling or morphogenetic outcome makes the genetic results hard to interpret.
Here we present a protocol that enables direct visualization of Furin-dependent signal cleavage by monitoring the spatiotemporal fates of the cleaved portions of a substrate in developing organs. The strategy and methodology was designed to examine Drosophila Bnl cleavage by Furin. The experiment takes advantage of the 3rd instar larval wing imaginal disc and an associated tracheal branch called the air sac primordium (ASP) (Sato and Kornberg, 2002). Bnl is expressed by a restricted group of wing disc cells and subsequently transported to the ASP to control its morphogenesis (Sato and Kornberg, 2002; Du et al., 2018). Bnl is cleaved by Furin-1 at the 164th amino acid residue prior to the interorgan transport of only its C-terminal truncated portion that contains the receptor binding site (Sohr et al., 2019). The truncated part of Bnl forms a characteristic long-range gradient in the ASP by binding to its receptor and traveling through actin-based filopodia or cytonemes extended by ASP cells (Du et al., 2018). To visually monitor the fates of the full-length Bnl and its truncated portions, we first generated a transgenic Drosophila line harboring a chimeric Bnl construct with an HA-tag at the 87th and a GFP tag at 432nd residue, flanking the cleavage site and conserved FGF domain. The construct was expressed in the wing disc Bnl source. Laval wing discs were dissected and ex vivo-cultured in the absence or presence of Furin protease inhibitors for variable amounts of time followed by α-HA immunostaining and fluorescence microscopic imaging of the discs. While in normal conditions only the C-terminal GFP-tagged fragment of Bnl was transported to the ASP, the addition of Furin inhibitors to the ex vivo culture resulted in uncleaved Bnl with both tags being transported to the recipient ASP cells (Figure 1). Since a broad range of signaling proteins involved in morphogenetic and disease pathways are modulated by Furin, the methodology described here can be a powerful tool to evaluate the role of Furin cleavage in various other signaling pathways. For instance, despite the wide-spread occurrence of signal cleavage modulating paracrine signaling activity, most full-length uncleaved forms of the signals were also found to activate receptors and are shown to be secreted when expressed in cultured cells (Künnapuu et al., 2009; Tulin and Stathopoulos, 2010; Sopory et al., 2010; Tokhunts et al., 2010; Constam, 2014). Therefore, a link between PC-dependent cleavage with the maturation and activity of the proteins remained obscure. The results from this assay together with other experiments showed that uncleaved Bnl is active and can be transported to the recipient cells to induce signaling. However, Bnl cleavage ensures its efficient polarized target-specific dispersion, thereby modulating its range and tissue-specific activity (Sohr et al., 2019). The ex vivo culture method with pharmacological Furin inhibition strategy is not limited to only the Drosophila wing imaginal disc and ASP tissue system. Similar assays can easily be applied to other Drosophila tissues, signaling proteins, or model systems.
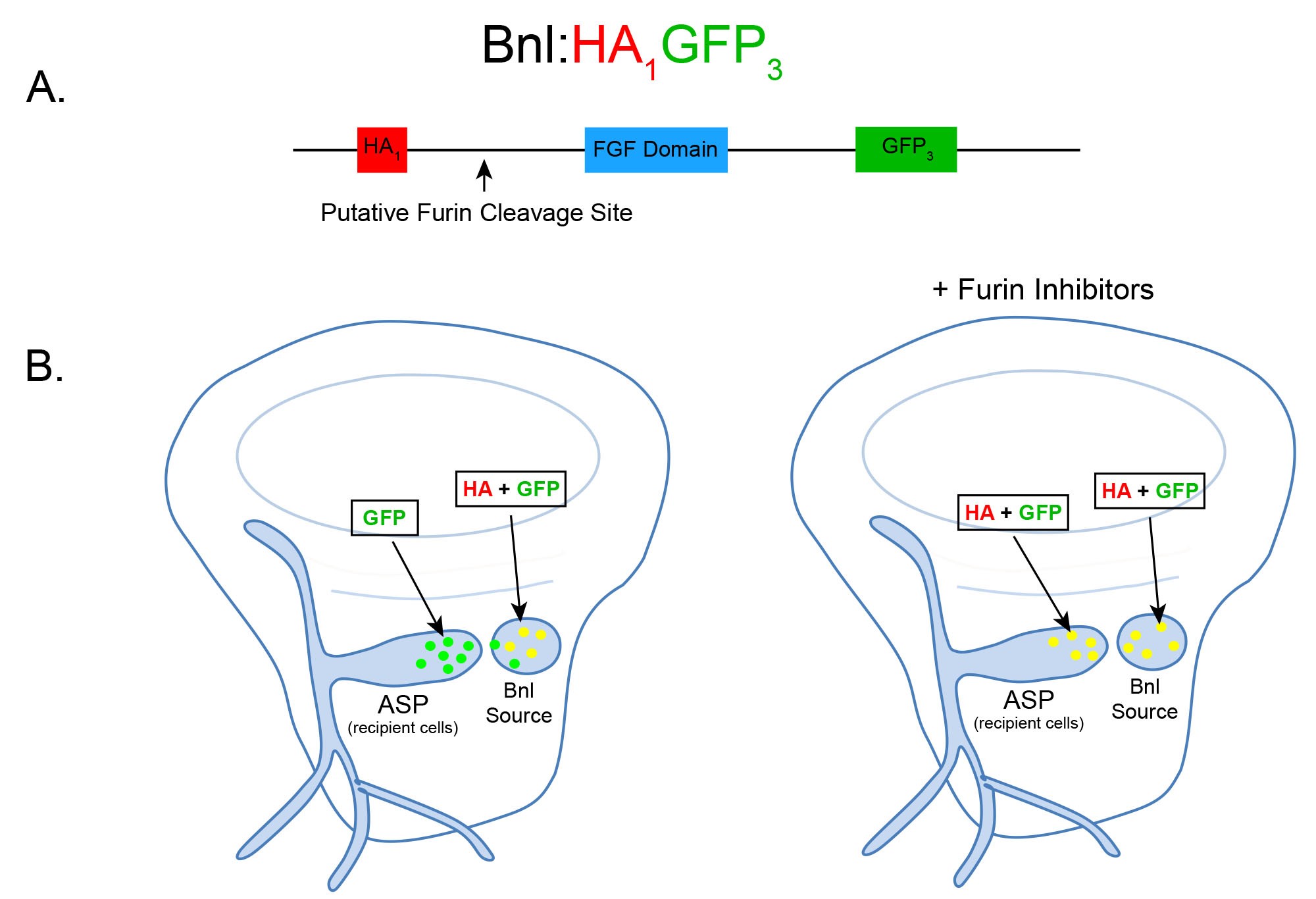
Figure 1. Schematic and expected dispersion patterns of Bnl:HA1GFP3. A. Schematic of the chimeric Bnl:HA1GFP3 protein, where an HA and GFP tag are located upstream and downstream of a Furin cleavage site, respectively. Bnl is cleaved at 164th amino acid and the HA and GFP tags are located at the 87th and 432nd residues, respectively. B. Cartoons of wing discs expressing Bnl:HA1GFP3 (yellow: GFP + α-HA red immunostaining) in the Bnl source. In ex vivo culture conditions without Furin inhibitors (left wing disc), Bnl is cleaved and only the GFP-tagged fragment of Bnl is transported to the ASP recipient cells. In ex vivo conditions with Furin inhibitors included, the amount of full length Bnl:HA1GFP3 (yellow) puncta in the ASP gradually increases with longer incubation time (right wing disc).
Materials and Reagents
- Drosophila rearing
- Fly Vials (Genesee Scientific, catalog number: 32-117)
- Vial Plugs (Genesee Scientific, catalog number: 49-101)
- Transgenic Drosophila lines: bnl:Gal4/TM6 and UAS-bnl:HA1GFP3 (Sohr et al., 2019)
- Standard Fly Nutrient Medium (Genesee Scientific, catalog number: 66-114)
- Propionic Acid (Sigma, catalog number: 402907)
- Late third instar larval dissection and washing
- Small paint brush (VWR, catalog number: 149-2130)
- Pipette tips (VWR, catalog number: 83007-376)
- Sterile Eppendorf tubes (Genesee Scientific, catalog number: 24-282)
- Pyrex spot plate (Fisher Scientific, catalog number: 13748B)
- 35 mm Plastic Petri dishes (Falcon, catalog number: 351008)
- Filter unit to sterilize WM1 media (VWR, catalog number: 89220-694)
- 100% ethanol (Pharmco, catalog number: 111000200)
- Deionized water
- Schneider’s medium (Sigma, catalog number: S0146)
- Insulin solution from bovine pancreas (Sigma, catalog number: I0516)
- Fly extract (DGRC, catalog number: 1645670)
- Penicillin/Streptomycin solution (Corning, catalog number: 30-002-CI)
- 70% ethanol
- WM1 media (Zartman et al., 2013) (see Recipes)
- Ex vivo culturing of dissected larvae
- Endotoxin-free water (Sigma, catalog number: 210-7)
- Furin inhibitor I (EMD Millipore, catalog number: 344930)
- Furin inhibitor II (EMD Millipore, catalog number: 344931)
- Phosphate buffered saline (PBS) (Hardy Diagnostics, catalog number: U137)
- Furin inhibitor cocktail (see Recipes)
- Antibody staining of larval carcasses
- 10 ml Syringe (Becton Dickinson, catalog number: 301029)
- Sterile syringe filter, 0.2 μm (VWR, catalog number: 28145-501)
- 16% formaldehyde (Ted Pella, Inc., catalog number: 18505)
- Triton X-100 (Sigma, catalog number: T8787)
- Normal goat serum powder (Jackson ImmunoResearch, catalog number: 005-000-121)
- α-HA antibody, 3F10 (Roche, catalog number: 11867423001)
- α-Discs Large antibody, 4F3 (DSHB, catalog number: AB_528203)
- Alexa Fluor 647 Goat anti-Rat IgG (Invitrogen, catalog number: A21247)
- Alexa Fluor 555 Goat anti-Mouse IgG (Invitrogen, catalog number: A21422)
- 4% formaldehyde (see Recipes)
- 0.1% PBS-T (see Recipes)
- 5% Normal goat serum in PBS-T (see Recipes)
- Mounting and imaging wing imaginal discs
- Microscope slides (Fisher Scientific, catalog number: 12-550-18)
- Coverslips (Corning, catalog number: 2845-22)
- Vectashield mounting medium (Vector, catalog number: H-1000)
- Nail polish (Electron Microscopy Sciences, catalog number: 72180)
Equipment
- Drosophila rearing
- Stereomicroscope (e.g., Standard Olympus SZ61 or Nikon SMZ745)
- Drosophila workstation with CO2 pad (Flowbuddy Complete; Genesee Scientific, catalog number: 59-122WC)
- Incubator (25 °C, Peltier Refrigerated incubator)
- Late third instar larval dissection and washing
- Dumont forceps #5 (Fine Science Tools, catalog number: 11252-00)
- Stereomicroscope (as described in section A)
- Pipette (1,000 μl)
- Scissors (VWR, catalog number: 89259-936)
- Ex vivo culturing of dissected larvae
- Sterile 25 °C incubator (Thermo Scientific, model: Heracell150i)
- Sterile biosafety cabinet for cell culture (Labconco, model: 302481101)
- Antibody staining of larval carcasses
Nutating mixer (VWR, model: 82007-002) - Mounting and imaging wing imaginal discs
- Forceps
- Laser scanning confocal microscope (e.g., Leica SP5X) or spinning disc confocal microscope (e.g., Nikon)
Software
- Imaging Software: IQ3 (Andor)
- ImageJ/Fiji
- Adobe Photoshop, Adobe Illustrator
- Microsoft Excel
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Sohr, A., Du, L. and Roy, S. (2019). Ex vivo Drosophila Wing Imaginal Disc Culture and Furin Inhibitor Assay. Bio-protocol 9(16): e3336. DOI: 10.21769/BioProtoc.3336.
- Sohr, A., Du, L., Wang, R., Lin, L. and Roy, S. (2019). Drosophila FGF cleavage is required for efficient intracellular sorting and intercellular dispersal. J Cell Biol 218(5): 1653.
分类
发育生物学 > 细胞信号传导 > 配体
细胞生物学 > 细胞成像 > 共聚焦显微镜
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link